
Article
Top 10 SaMD Job Boards & Regional Organizations
This blog contains Chapter 1 & 2 of the Orthogonal eBook titled: Digital Therapeutics (DTx): Accelerating Success Using Fast Feedback Loops. The following are links to each chapter of this eBook:
Acknowledgments, and why this is a “living” eBook
While the content, errors and omissions in this document are the sole responsibility of the authors, Orthogonal thanks the many industry experts who have provided their invaluable insights and feedback to us along the way. This august list includes:
We refer to this as a “living” eBook because in a domain as nascent and yet already so complex as DTx, no attempt to summarize the state of the market will ever be complete. There will constantly be evolutions to the ideas, companies and therapeutics mentioned in this paper. New players, research, best practices, and worst practices will emerge. There will be references, ideas and predictions in here that don’t stand the test of time.
Finally, in our efforts to keep this document to under 60 pages, we made countless tradeoffs between diving deeper, going broader and preserving a straightforward narrative. (As opposed to letting this drift into document the length of a Victor Hugo novel.) In the process, we have left many topics and details, and tried to simplify many, many others while still being accurate.
We want to hear from you! What is missing from this document? What should get more attention? What did we say that you agree with? Even more importantly, what did we say that you disagree with? (Typos?)
We look forward to adding your name to the above acknowledgment list, or having you join the ranks of anonymous contributors to this document. Our contact information is at the end of this document.
Medicine is embracing the full power of technology. Technology integration started with the electronic health record and then transitioned to patient engagement, streamlining the patient journey as patients raised their consumer voices demanding a better, less-convoluted, and frustrating experience. The industry is now realizing that the power of technology reaches far beyond a well-designed patient portal and patient-facing mobile application. Technology can be medicine. More specifically, software code can be medicine.
What sounds like a sci-fi future state to some, is already a full-fledged reality for others. The technological shift underlying the adoption of digital therapies is powered by the Internet of Things, or IoT,[1] a growing network of physical objects that communicate using the internet. In addition to IoT, new standards promoting interoperability in the healthcare space, such as the HL7 Fast Healthcare Interoperability Resources (FHIR) standard,[2] have eased integration and data analysis, supporting the more seamless integration software into patient care journeys.
The information from IoT devices and medical sensors drive healthcare innovation when it is collected by software applications and combined with user data. Known as Software as a Medical Device (SaMD), this category of software helps prevent, manage, or treat medical conditions without actually being part of a hardware medical device.
SaMD enables a new class of digital products acting as therapies to treat conditions and diseases. Digital therapeutics (DTx) deliver evidence-based interventions to patients that are similar to more traditional therapies involving physical interactions with the human body. Compared with traditional pharmaceutical drug development, DTx can be created via quickly iterative and rapidly progressing technology. Fast feedback loops and proven software iteration processes, such as the agile methodology, used in DTx development support fast-paced improvement, as well as personalization.
As this eBook will discuss, MedTech firms that manage their DTx-like software with fast feedback loops and a hyper-focus on iteration will greatly increase their odds of success. Skeptics may think rapid innovation and iteration cannot possibly co-exist within an FDA-regulated product. And, although it is true that the tech rallying cry to “move fast and break things” cannot be flippantly followed because patient safety is at stake, “moving faster and breaking nothing” is well within reach. In Orthogonal’s e-book, Agile in an FDA Regulated Environment, we discuss how health-tech companies can move fast intentionally and thoughtfully. In this eBook, we delineate the business and societal imperative of following this approach to delivering the full value of DTx.
One final introductory note: At Orthogonal and in this eBook, we regularly refer to the following four lenses of value that can be created by DTx. We’ve summarized them here as a reference as you read through the rest of this eBook:
A therapeutic (Tx) can refer to a drug, medical device, or software that helps prevent, treat, or manage a disease or condition.[4] A digital therapeutic (DTx) is a subcategory of Tx in which the administration of the therapy has been digitized. Specifically, the digitized therapy is delivered to the patient via an interaction with a computer, as opposed to via a pill, injection, or face-to-face interaction with a clinician. The Digital Therapeutic Alliance states: “Digital therapeutics (DTx) deliver medical interventions directly to patients using evidence-based, clinically evaluated software to treat, manage, and prevent a broad spectrum of diseases and disorders.”[5]
Like many new concepts and disruptive healthcare interventions, gaining a complete picture of DTx and its potential can be challenging. To better illustrate the concept of DTx versus other categories of Tx, Table 1 provides a high-level overview and examples of prominent categories of traditional Tx and DTx.
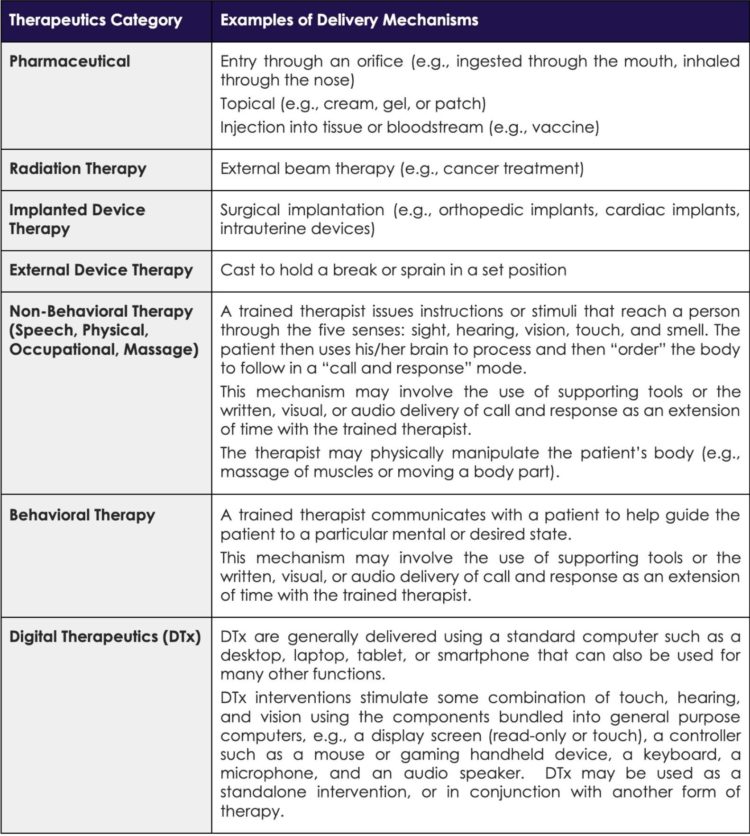
Example Categories of Therapeutics (Tx)
In practice, early DTx have primarily focused on behavioral therapies that can augment or replace the work of a trained clinician (i.e., physician, nurse, or allied health professional). These DTx involve some form of active coaching or guidance to a patient that induces behavior changes linked to improved health outcomes. DTx can be administered as standalone interventions, or they can be combined with other forms of interventions including drugs, medical devices, and behavioral and non-behavioral therapies.
As with any other type of therapy, for a DTx to be approved by the FDA or other national government regulators and to be reimbursed by insurers (e.g., CMS, CIGNA, BCBS), the manufacturer needs to prove that the therapy is safe, provides a demonstrable health benefit (i.e., it is clinically effective), as well as achieves a return on investment (ROI) that justifies its use in specific situations versus another therapy or no treatment at all (i.e., it is comparatively effective).
DTx, digital medicine, and digital health all have the goal of better human health and are delivered through connected devices such as mobile apps, desktop web browsers, and IoT-enabled devices. Although similar in form, the function and measurable outcome of each varies.
An excellent set of definitions (Fig. 1) with clear examples (Fig. 2) has been created by a consortium of the Digital Medicine Society (DiMe), Digital Therapeutics Alliance (DTA), HealthXL, and NODE.Health.[6] This team used a risk-based framework and described the relevant areas of regulatory oversight for each of the three categories they defined.
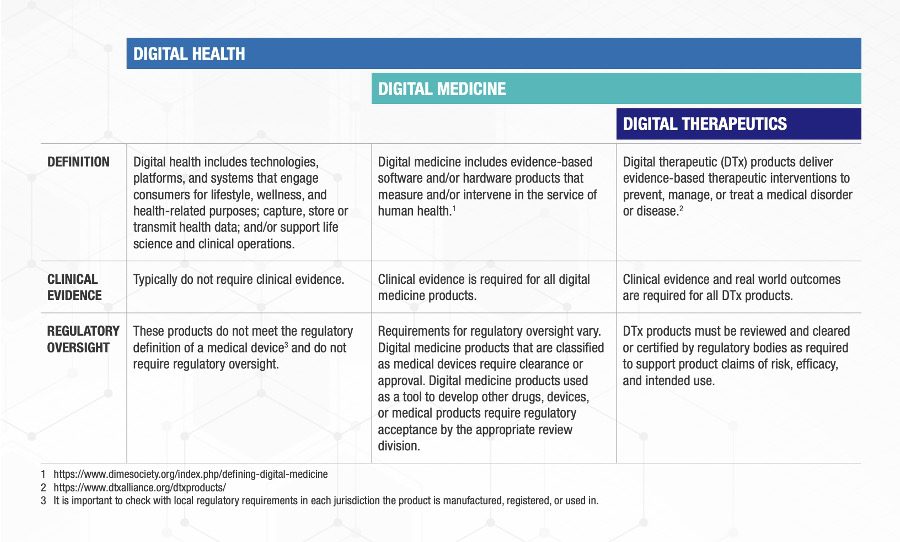
Fig. 1: The consortium’s summary of the evidentiary requirements for and regulatory oversight of different digital health products.
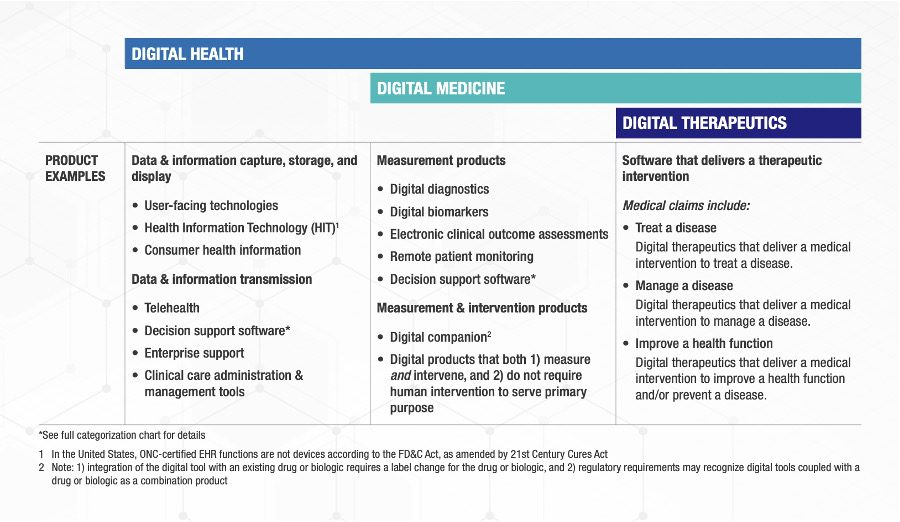
Fig. 2: The consortium’s examples products/services for the three categories
The consortium’s framework classifies digital wellness as a part of digital health. However, the examples under the Digital Health column in Fig. 2 do not include products such as iOS and Android apps in app stores that can be downloaded by consumers for general wellness such as applications to assist with weight loss, teach yoga, or train runners.
For the purposes of this eBook, we believe that these applications are more appropriately bundled into a fourth category of mostly unregulated products for Digital Wellness[7] that are intended to have Tx value. Thus, expanding on the consortium’s framework, and encompassing the advent of DTx, a fourth category of Digital Wellness is recommended as shown in Fig. 3.
Although digital wellness apps can help users improve their health by assisting them in tracking their weight, optimizing nutritional intake, or spending less time on their cell phone, typically, a digital wellness product does not have to prove safety or effectiveness before it can be allowed on the market in the U.S.
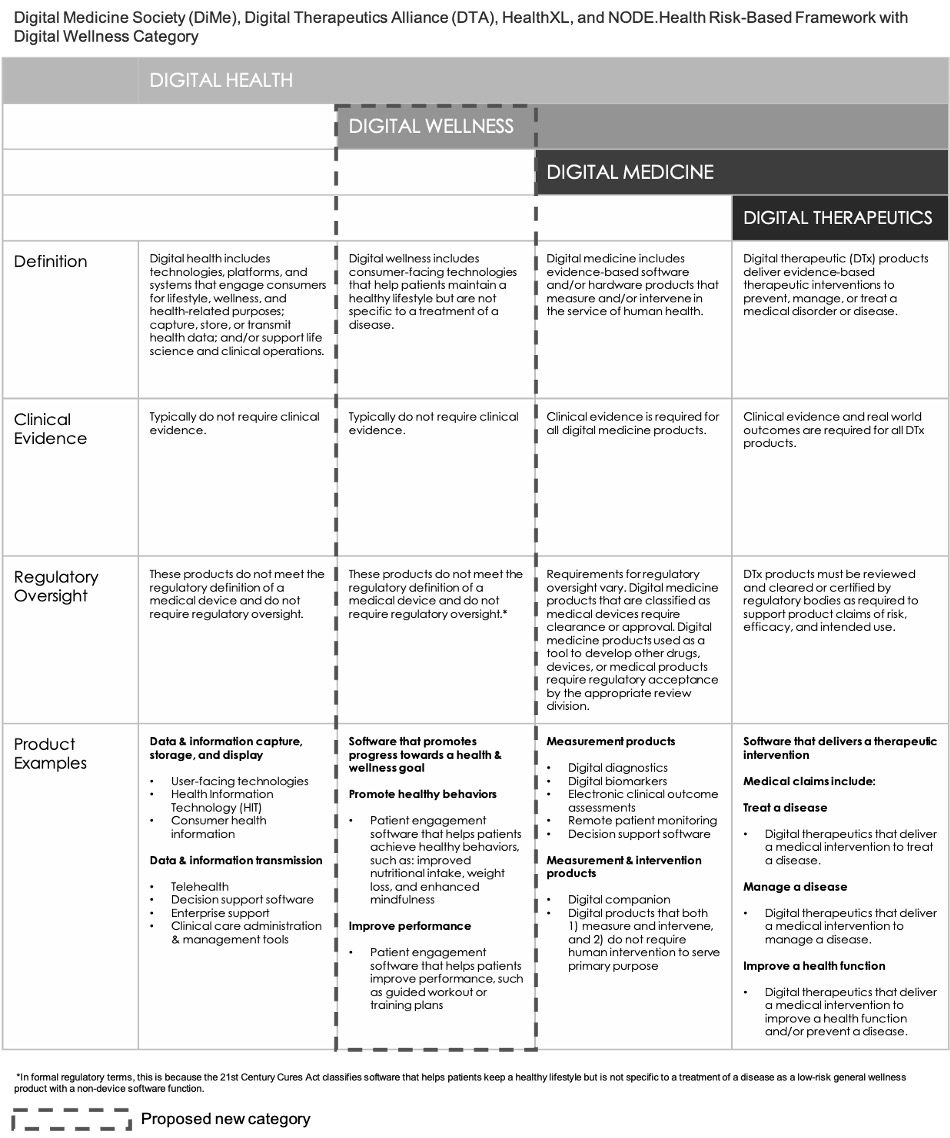
Fig. 3: Orthogonal’s Proposed Update to the Consortium Framework from DiMe, DTA, HealthXL and NODE.Health
Orthogonal is a software developer for Software as a Medical Device (SaMD), Digital Therapeutics (DTx) and connected medical devices.
We work with change agents who are responsible for digital transformation at medical device, diagnostics, and pharmaceutical manufacturers. These leaders need to accelerate their pipeline of product innovation to modernize patient care and gain a competitive advantage.
Orthogonal applies deep experience in SaMD and the power of fast feedback loops (FFLs) to rapidly develop, successfully launch, and continuously improve connected, compliant products—and we collaborate with our clients to build their own SaMD development engines.
Over almost a decade of work, we’ve helped a wide variety of MedTech firms develop and bring their regulated/connected devices to market.
Almost twenty years ago, we began our consulting work to create great software products in a range of industries, including financial services, education, research, and healthcare. We spent nearly a decade working with leading-edge FFL techniques that have now become recognized best practices such as Agile software development (with XP, Scrum, and Kanban), Lean User Experience (Lean UX), DevOps and Lean Startup.
Nearly a decade ago, we got a glimpse of digital healthcare and the enormous potential of the cloud, IoT, and smartphones, and the potential impact of medical device software. Realizing that’s what we wanted to focus our energies on exclusively, we narrowed our focus to the development of SaMD, DTx and connected medical devices.
We’ve never looked back. Our team gets up every day excited to help move the needle on healthcare. For almost ten years, we’ve helped a wide variety of firms develop and bring their regulated/connected devices to market. We’re even more excited about the next ten years and what we will help accomplish during The Great Acceleration of Digital Health.[8]
If you need help building your next SaMD, or to learn more, send us a message or call us at (866) 882-7215.
Social Media
Bernhard Kappe, CEO and Founder
Bernhard Kappe is a software development leader, technologist, and pioneer who consistently provides his clients with an unfair advantage by introducing them to important new trends and techniques at the leading edge of the adoption bell curve (e.g., Agile software development, Lean User Experience, Software Product Management, DevOps, Lean Startup and open-source software).
For the last eight years, as founder and CEO of Orthogonal, Kappe has exclusively focused on adapting these approaches to the medical device and HealthTech industry.
He has worked with clients including Novo Nordisk, Google, Tandem Diabetes, and Nanowear to connect medical devices to mobile apps and the cloud while shortening product development life cycles, improving delivery predictability, and creating engaging user experiences. By developing new diagnostic and treatment devices and business models, his clients have seen software-enabled market growth and improved patient outcomes and have contributed to bending the healthcare cost curve.
Bernhard can be reached via:
Email: bkappe@devorthogonal.wpengine.com
LinkedIn: bernhardkappe
Twitter: @bernhardkappe
Phone: 312-372-1058 x 6002
Randy Horton, VP of Solutions & Partnerships
Randy Horton is VP of Solutions and Partnerships at Orthogonal, a software development consulting firm that gives medical device manufacturers an unfair advantage by helping them rapidly design and develop new Software as a Medical Device (SaMD) and connected medical device solutions. Randy Horton is passionate about helping established organizations break through to their “what’s next” by building new capabilities and launching new products and services that are both innovative and successful. Much of his career has been centered on working with healthcare and life sciences organizations to tackle the problems summarized in the Quadruple Aims: Improving the individual experience of care, improving the health of populations, reducing the per capita costs of care, and improving the work life of those who deliver care. Randy credits much of his passion for creative business thinking and being a connector of people and ideas to the four years that he spent at a Montessori pre-school. Noting that he still seems to live in the open classroom of his childhood, one of Randy’s executives once observed, “It’s not that Randy thinks out of the box. It’s that he doesn’t even have a box.”
Randy can be reached via:
Email: rhorton@devorthogonal.wpengine.com
LinkedIn: randyhorton
Twitter: @randyhortonchi
Phone: 312-433-9437
Bryana Banashefski, Digital Health and SaMD Device Strategist
Bryana Banashefski serves as a part-time Digital Health and Software as a Medical Device Strategist at Orthogonal. She has 6 years of experience in the healthcare technology industry and has served as a Product Manager building patient engagement software for global pharmaceutical firms and national healthcare systems. Her career north star is using technology to improve patient outcomes and reduce healthcare inequities. She is a second-year medical student at the Icahn School of Medicine in New York City in the pursuit of getting closer to patients and helping to fix the healthcare industry inside the belly of the beast. Bryana hopes to help bridge the gap between the health-tech industry and the frontlines of healthcare delivery and is excited to be a small part of building the tech-enabled healthcare of the future.
Bryana can be reached via:
Email: bbanashefski@devorthogonal.wpengine.com
LinkedIn: bryanabanashefski
Twitter: @bbanashefski
Phone: 713-315-7189
Financial Disclosures for the Authors
This blog contains Chapter 1 & 2 of the Orthogonal eBook titled: Digital Therapeutics (DTx): Accelerating Success Using Fast Feedback Loops. The following are links to each chapter of this eBook:
References for Chapter 1 & 2 1. Burgess M. What is the Internet of Things? WIRED explains. WIRED UK. https://www.wired.co.uk/article/internet-of-things-what-is-explained-iot. Published 2018. [Accessed August 24, 2021]. 2. Overview - FHIR v4.0.1. Hl7.org. https://www.hl7.org/fhir/overview.html. Published 2019. [Accessed August 24, 2021]. 3. Moll B, Yew K. White Paper: Accelerating SaMD and DTx with Product Analytics. Orthogonal. https://devorthogonal.wpengine.com/insights/accelerating-samd-and-dtx-with-product-analytics/. Published 2021. [Accessed September 29, 2021]. 4. The terms treatment, therapy and therapeutic can have different definitions but are often used interchangeably. Therapy comes from a Latin root word meaning “medical treatment of disease.” Therapeutics comes from a Greek word meaning “to cure, treat medically.” The acronym Tx generally means treatment and not therapy, but because “digital therapeutics” is termed “DTx,” in this paper, the acronym Tx stands for “therapeutic.” 5. Digital Therapeutics Alliance. 2021. Understanding DTx - Digital Therapeutics Alliance. [online] Available at: <https://dtxalliance.org/understanding-dtx/> [Accessed 19 August 2021]. 6. Goldsack J, Coravos A, Coravos A, Navar-Mattingly N, Fitzgerald C, Coder M. Digital health, Digital Medicine, Digital Therapeutics (DTX): What's the difference? Digital Medicine Society (DiMe). https://www.dimesociety.org/digital-health-digital-medicine-digital-therapeutics-dtx-whats-the-difference/. Published November 10, 2019. [Accessed September 9, 2021]. 7. Waterfield S. Is 'Digital Wellness' Really The Answer? Forbes. https://www.forbes.com/sites/pheewaterfield/2018/06/06/is-digital-wellness-really-the-answer/?sh=1c67162a451c. Published 2018. Accessed August 23, 2021. 8. COVID-19 and "The Great Acceleration of Digital Health." Orthogonal. https://devorthogonal.wpengine.com/insights/covid-19-and-the-great-acceleration-of-digital-health/. Published 2020. [Accessed September 21, 2021].
Related Posts

Article
Top 10 SaMD Job Boards & Regional Organizations

Article
Top 10 SaMD Events, Conferences & Webinars

Article
Top 10 SaMD Publications, Blogs & Media

Article
Top 10 SaMD Communities, Organizations & Working Groups