
Article
Roundup: Bluetooth Medical Devices Cleared by FDA in 2024
This post was previously on the Pathfinder Software site. Pathfinder Software changed its name to Orthogonal in 2016. Read more.
One of the trends from the 2012 MHealth Summit I briefly touched on last week was the growing number of Bluetooth and wifi enabled wearable medical devices. I think this trend will become extremely important over the next few years.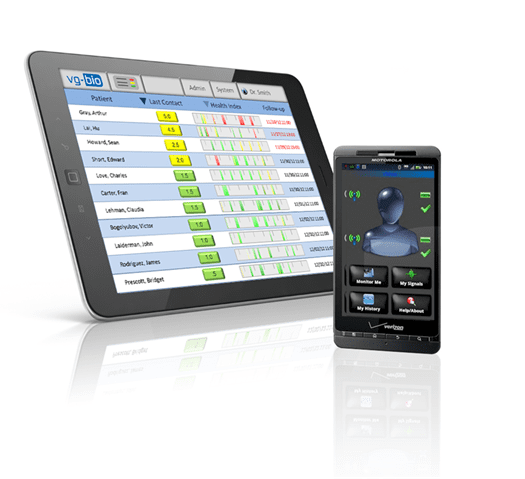
Body Area Sensor Networks combine pervasive wireless networks, small non-invasive sensors, and ultra-low power consumption chips to enable the continuous collection of physiology data from ambulatory patients.
VitaLink, a project we recently worked on with Insight Product Development and our client VGBio, illustrates the potential power of combining body area sensor networks with mobile apps, cloud computing and machine learning based predictive analytics.
The Vitalink remote patient monitoring solution consists of:
This technology enables the daily monitoring of patients with chronic diseases and provides early notification to clinicians of a negative change.
We’ve only scratched the surface of where we can go with mobile, wearable sensors and big data.
In the future, I can see the data this proactive monitoring system collects can also be combined with data from other sensors like activity monitors, intake monitors, weight and sleep monitors, as well as information on prescribed medications to help provide feedback on the effectiveness of different approaches to improving outcomes.
Related Posts

Article
Roundup: Bluetooth Medical Devices Cleared by FDA in 2024

Article
Help Us Build an Authoritative List of SaMD Cleared by the FDA

Article
SaMD Cleared by the FDA: The Ultimate Running List

Article
Roundup: Bluetooth Medical Devices Cleared by FDA in 2023