
Article
Studying Compliance Burden to Improve SaMD Development

The entire Orthogonal team offers a huge congratulations (and thanks!) to Nanowear and its supporters and investors[1] for launching a groundbreaking COVID-19 research project in partnership with two major health systems in the NY Metro region:
This clinical trial will remotely monitor patients with confirmed or suspected COVID-19 using SimpleSENSE, Nanowear’s one-size-fits-all adjustable undergarment. In this trial, Nanowear’s clinical-grade MedTech device will detect physiological and biomarker changes indicative of clinical deterioration that may require further intervention from the hospital systems.
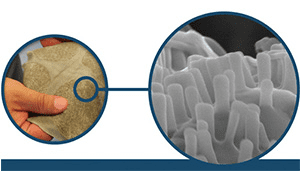
Nanowear’s SimpleSENSE, the first cloth-based nanotechnology monitoring platform to receive 510(k) clearance from the FDA, provides a great platform to fill this urgent clinical need. When a patient wears it, physicians can remotely capture and assess multiple physiological signals—including real-time ECG, systolic and diastolic blood pressure, blood flow hemodynamics, respiration, lung volume and fluid, and temperature trends—without the need for an in-person visit or physical touch.

Nanowear Company Overview from Nanowear on Vimeo.
Orthogonal is grateful to have had the opportunity to work with Nanowear on the original software development for their continuous ECG monitoring solution. Orthogonal collaborated with Nanowear to apply Orthogonal’s agile life cycle product development approach to developing the software and optimizing the Bluetooth connectivity that allows a smartphone app to continuously capture the nanosensor-generated data monitoring from Nanowear’s cloth-based monitoring devices.
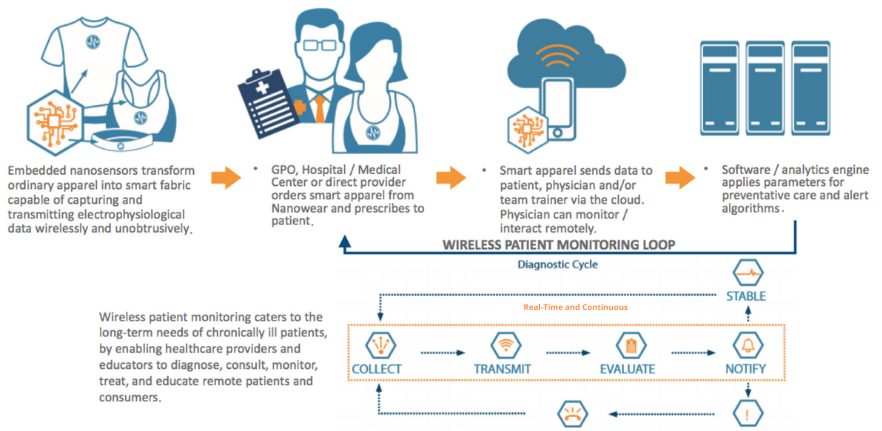
For this work, Orthogonal applied interface control processes and a lightweight testing and simulation framework, enabling rapid parallel development of the device and software. This enabled faster build-test-fix cycles for both hardware and software by surfacing system-level issues early.
In light of the ongoing severity of the COVID-19 pandemic, the team at Orthogonal is particularly excited and gratified to see how this solution has continued to be adapted to address important clinical needs, specifically using digital health to close crucial care delivery gaps arising from this global outbreak.
The validation of this new application of this technology is a terrific example of how COVID-19 is driving The Great Acceleration of Digital Health. We agree wholeheartedly with their assessment of how connected mobile medical devices will fuel the long-term growth of telemedicine as a part of the care continuum:
On a more personal level, we at Orthogonal feel truly lucky to have been able to take part in the early phases of a groundbreaking medical product that has the potential to play a key role in the fight against this virus. Specifically,
As New York City, the epicenter of the global coronavirus pandemic, emerges from coronavirus lockdown, health systems are looking for novel technologies like Nanowear to better understand and combat the unprecedented severity of COVID-19. Nanowear is being used to aid in COVID-19 diagnosis to ensure the health and safety of patients and medical professionals.
We look forward to reading more about the results of this clinical trial. We expect to see great things continue to emerge from Nanowear and other pioneers in the connected mobile medical device (CMMD) and Software as a Medical Device (SaMD) space.
Orthogonal is a software developer for connected mobile medical devices (CMMD) and Software as a Medical Device (SaMD). We work with change agents who are responsible for digital transformation at medical device and diagnostics manufacturers. These leaders and pioneers need to accelerate their pipeline of product innovation to modernize patient care and gain competitive advantage.
Orthogonal applies deep experience in CMMD/SaMD and the power of fast feedback loops to rapidly develop, successfully launch, and continuously improve connected, compliant products—and we collaborate with our clients to build their own rapid CMMD/SAMD development engines. Over the last eight years, we’ve helped a wide variety of firms develop and bring their regulated/connected devices to market.
Bernhard Kappe is Founder and CEO at Orthogonal. You can email him at bkappe@devorthogonal.wpengine.com
Randy Horton is VP of Solutions and Partnerships at Orthogonal. You can email him at rhorton@devorthogonal.wpengine.com
**
Footnotes
Related Posts

Article
Studying Compliance Burden to Improve SaMD Development

Article
Lifestyle Integration for Medical Devices

Article
CEOs and other Digital Health Experts on SaMD in 2020

Article
Looking Back at a Pioneer in DTx and SaMD – Propeller Health in 2013