SaMD = Software & Medical Device
Getting medical device quality activities moving at an Agile development pace.
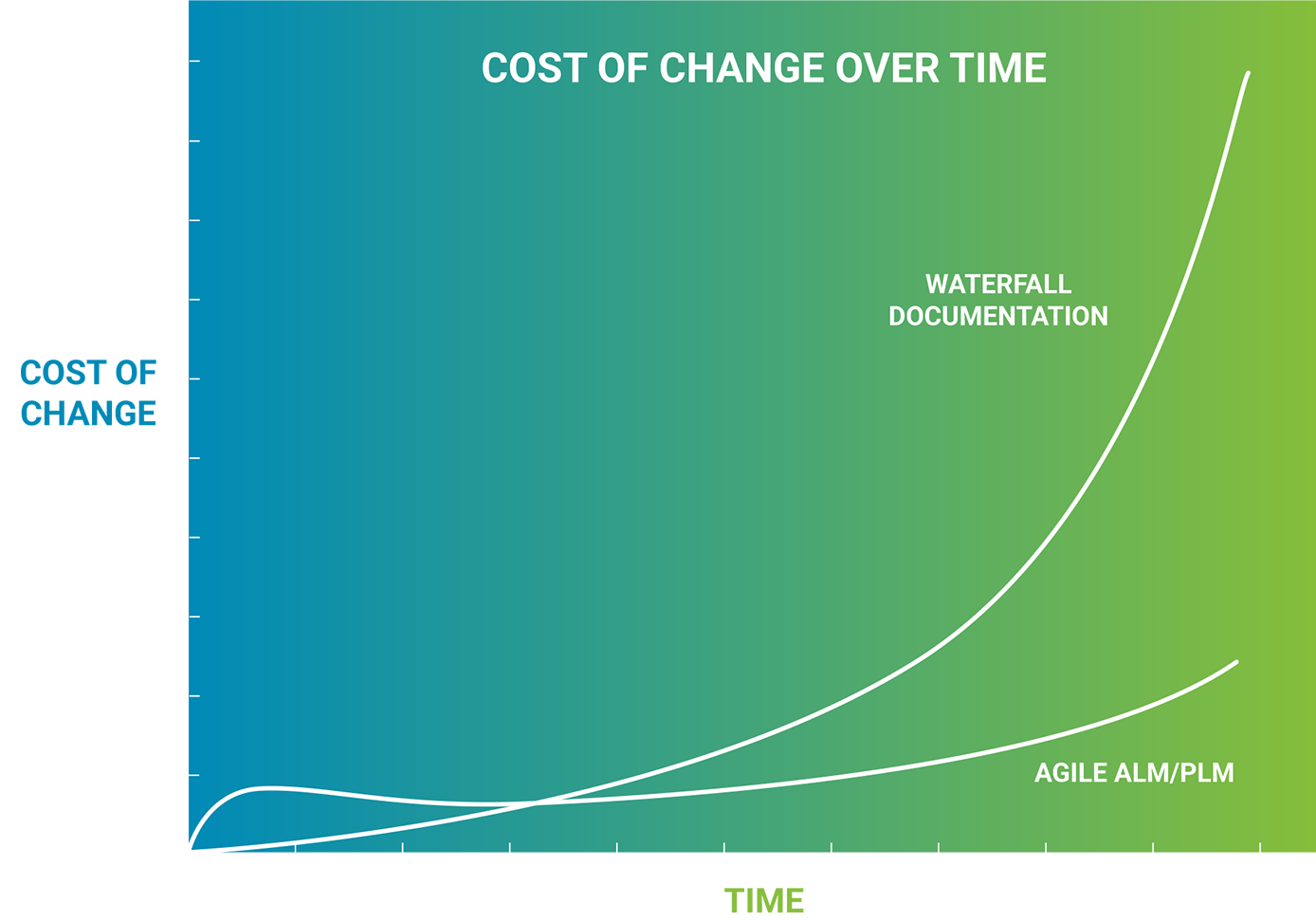
Agile development moves quickly, as developers work in short increments to create resilient and adaptable software. Medical device regulations were written in a time before Agile and the internet, and assume waterfall development. Bringing these two worlds together can create an impedance mismatch – more work, less adaptation to change and little to no gain in safety, compliance or outcomes. At Orthogonal, we believe the solution to this impedance mismatch is making non-development activities more Agile, and we accomplish that with our electronic Quality Management System (eQMS).
Our eQMS
Integrating ALM & PLM into familiar tools to save time & improve quality
Jira and Confluence are software development tools that many teams already use effectively. That’s why we built our Application Lifecycle Management (ALM) and Product Lifecycle Management (PLM) features on top of them, allowing us to rapidly generate, reuse and automate requirements, risk management, specification, verification and traceability documentation. Our eQMS improves the quality of our software products while reducing the cost and duration of design controls activities by 25–50% – and enables our customers to release faster without increasing regulatory and quality concerns.
Automated Traceability on Jira
Trace requirements, manage risks and automate tests right where development happens.
User stories are at the heart of Agile SaMD development. Our eQMS’s add-ons to Jira simplify managing and fulfilling those stories. We can manage, track and trace requirements, and build the traceability matrices for user needs, software requirements, verifications and other structures within Jira. Risk management is handled through matrices and tables, allowing us to do risk analysis compliant with ISO 14971 – including cybersecurity risk. A well-organized repository of manual or automated tests makes verification simple and mitigation easy to trace. Combined, we use all of these add-ons to develop, test and verify user stories in one place.

Automated Documentation on Confluence
Speed compliance and approval with dynamic documentation.
Traditional QMS rely on physical documentation done by hand. Our eQMS’s add-ons to Confluence allow us to automate documentation, speeding compliance and approval without additional work. We’re able to review and approve documents in tailored workflows with Part 11-compliant signatures, maintain both working copies and publish approved or in-effect documents, and freeze and approve dynamic content for document approval. Changes in Jira feed into Confluence documentation, allowing flexibility while maintaining compliance protocols.

Your SaMD, Your eQMS
Accelerate development with our comprehensive, intuitive and compliant system.
Don’t let documentation and compliance protocols slow down your SaMD development. Orthogonal can tailor our eQMS to meet your product and teams’ needs, allowing you to hit crucial milestones, funding and regulatory deadlines and market launches.

