
Article
Roundup: Bluetooth Medical Devices Cleared by FDA in 2024
We owe a lot to the earliest Bluetooth-enabled medical devices that successfully gained FDA clearance.
These medical devices – and their manufacturers – were the first to demonstrate how Bluetooth technology could augment the use and functionality of medical devices. They laid out the groundwork upon which powerful, sophisticated Bluetooth-connected devices would later be built. Even today, in the absence of definitive FDA guidance for Bluetooth-enabled devices, MedTech manufacturers continue to draw from the precedents they set.
While the lack of clear FDA guidance is vexing, these examples show that obtaining clearance is possible. For those seeking advice on meeting FDA requirements for Bluetooth connected devices, specifically their mobile medical app components, you can watch a recording of our webinar “Meeting FDA Requirements for BLE Mobile Medical Apps.” Orthogonal and MedSec hosted this webinar on September 20, 2023, as part of our ongoing Bluetooth Low Energy for Medical Devices‘ series.
Basil Systems, an online database of healthcare information, was essential in researching this article. Shout out to them and their highly navigable, easy-to-understand tool that makes reviewing FDA databases a breeze.
One of the first Bluetooth-enabled medical devices intended for patient use is the Wireless Medicine LifeSync System from GMP Companies, Inc, released in 2003. This multi-part device included a cordless ECG monitor that converted waveforms into digital data and communicated over Bluetooth with a monitor transceiver. The transceiver then reconstructed the waveforms and displayed them on a compatible ECG system, making those systems wireless as well.
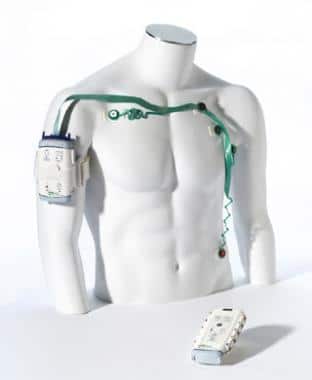
Source: IDSA
The novelty of this ECG monitor was in its lack of wires, as well as its “friendly” and “less threatening” design, according to the Industrial Designers Society of America (IDSA), who presented an International Design Excellence Award to the device in 2005.
While modern medical devices are known for their portability, this device seems intended for use on a stationary patient. IDSA’s award description mentions it being useful in a “chaotic ER,” though it could also have been used in a patient’s home, assuming they have a compatible ECG system.

Source: Vitalograph
The A&D Medical UA-767 BT Digital Blood Pressure Monitor, cleared in 2004, is, as the name suggests, a device that monitors a patient’s blood pressure. It used a traditional inflatable cuff to take a blood pressure measurement, which was then displayed on the device’s LCD screen and sent over Bluetooth to compatible devices. This being 2004, the list of compatible devices included a PC, a printer and even a PDA. Anyone got a Palm Pilot handy?
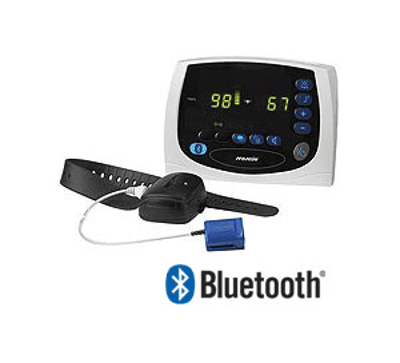
Source: Surgical Discounters
Today, you can buy a pulse oximeter that includes the digital readout within the oximeter itself. But back in 2004, Nonin Medical’s Avant Model 4000 Digital Pulse Oximetry System, with its watch-like oximeter module and desk-mounted tabletop display, was cutting-edge.
The Bluetooth connection between the oximeter module and the display gave patients the “increased ability to move freely without being hindered by cables,” according to the summary document from the FDA’s database.
Interestingly, this device names the previously discussed Wireless Medicine LifeSync System as its predicate for a Bluetooth-enabled device. It’s a great example of how Bluetooth devices build upon the success of their predecessors.
Before continuous glucose monitors (CGMs) hit the market (more on that later), a company called e-San LTD received clearance in 2005 for their Bluetooth Cradle. It was an accessory to LifeScan’s OneTouch R Ultra Blood Glucose Monitoring System. According to the FDA summary, the cradle plugged into the OneTouch’s stereo socket and used Bluetooth V1.2 (!) to transmit data to cell phones, such as the Nokia Model 6230.

Remember when this was top of the line? Now I feel old.
Source: Wikipedia
While this device (and its manufacturer) left little mark on the Internet, it’s an interesting snapshot of a predecessor to the modern CGMs.
Jumping forward to 2012, a time when cell phones evolved into smartphones, and when Reciprocal Labs received FDA clearance for their Asthmapolis System. It combined an electronic sensor that sat on top of a metered dose inhaler (MDI) and recorded usage with a mobile app that received signals from the sensor and transmitted them to a remote storage system. It also provided novel geographic and temporal tracking, building a map of where and when patients used their MDI to help spot triggers and patterns in symptoms.

Source: MobihealthNews
Reciprocal Labs transformed into Propeller Health in 2013. They’ve continued their pioneering work into facilitating the treatment of asthma and COPD with connected medical device systems to this very day. For more on Propeller, read our article on them from 2021.
One of the earliest if not the earliest medical devices to use Bluetooth Low Energy (BLE) is the MedApps 2.0 Remote Patient Monitoring System from 2013, three years after BLE was introduced. Serving as a combination physical data hub and companion web-based health data management application, it’s another early connected medical device.
Like the e-San Bluetooth Cradle, the physical part of this device plugged into already existing medical devices (such as glucose meters, blood pressure monitors and oximeters) and wirelessly transmitted data, delivering it to the companion web app for clinicians to review.

The MedApps HeathPal was a component of the 2.0 Remote Patient Monitoring System
Source: MobihealthNews
MedApps covered their bases when it came to wireless connectivity options. According to the FDA, the device was not only compatible with BLE, but also with Bluetooth Classic, WiFi, Zigbee and Fitlinxx, an “exercise workout tracking system” from fitness company ActivLinxx.
MedApps was acquired by point-of-care diagnostics company Alere in 2012 (hence why Alere’s name is included in the FDA’s summary). Alere was then acquired by Abbott in 2016.
In 2015, Dexcom received FDA clearance for their G4 Platinum Continuous Glucose Monitoring (CGM) System with Share, a BLE receiver. Though it was one of the first CGMs, it was not Dexcom’s first foray into BLE for the G4 system.
One year earlier, they received clearance for a BLE-enabled charging cradle, also called Share. This device was both a charging station for the Dexcom G4 CGM as well as a BLE receiver, with a range of 20 feet. It shared data from one CGM with multiple smartphones, making it easy for clinicians and families to track the glucose levels of a patient, child or spouse.
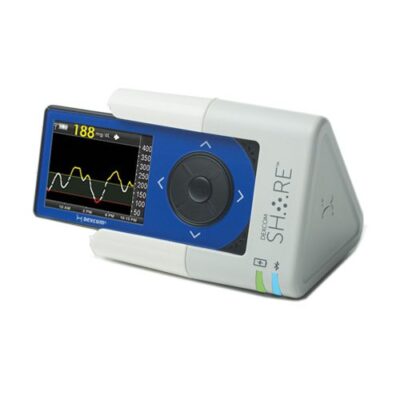
Source: MobihealthNews
Though the Share Charging Cradle was quickly replaced by the receiver (with Dexcom offering free upgrades to owners of the former to the latter), it holds an interesting place in the transition to the CGMs that have been widely adopted by people with diabetes today.
_______
Looking to solve the biggest BLE challenges for your connected medical device? Orthogonal and MedSec’s joint Bluetooth Low Energy for Medical Devices Webinar Series shares best practices for the successful implementation of Bluetooth for connected medical device systems. Register to attend an upcoming webinar or join the mailing list for relevant content sent straight to your inbox.
Related Posts

Article
Roundup: Bluetooth Medical Devices Cleared by FDA in 2024

Article
Roundup: Bluetooth Medical Devices Cleared by FDA in 2023

Article
Testing Strategies for Bluetooth Medical Devices

Article
Patient Engagement & UX for Bluetooth Medical Devices