
Article
Help Us Build an Authoritative List of SaMD Cleared by the FDA
Connecting a medical device over Bluetooth to a companion app on a patient’s smartphone opens up new possibilities for device manufacturers. In 2023, Orthogonal and MedSec’s joint Bluetooth Low Energy for Medical Devices Webinar Series dove into best practices for successfully implementing Bluetooth connectivity in medical devices. Our expert insights are available to read in our Bluetooth for Medical Devices White Paper.

Though our webinar series has concluded, we’re still keeping an eye on recent Bluetooth-enabled medical devices that have received FDA clearance. What Bluetooth medical devices have been cleared by the FDA in 2024 so far? This blog lists devices that have made their clearances public and describes how they use Bluetooth.
_________________________________________________________________________
January 4th, 2024

Source: Koya Medical
Dayspring is a wearable compression system designed to be worn on the arm or leg. It is used to increase lymphatic flow, which aids in the treatment of conditions like lymphedema and edema. The device consists of a physical compression garment, a controller attached to the garment, and a mobile app. The treatment provided by the garment can be customized through a mobile app connected to the controller over Bluetooth. Patients can also adjust settings and view their treatment history on the app.
January 18th, 2024

Source: NuvoAir
NuvoAir’s Air Next is a handheld spirometer that performs basic lung function and spirometry testing for use in hospitals, clinical settings and the home. When patients breathe into the device, a digital infrared interruption software counts the number of rotations and converts that measurement into airflow measured in liters per second. Test results are shared with patients over Bluetooth on the Air Next app.
January 31, 2024

Source: Masimo
The MightySat OTC from Masimo is a fingertip pulse oximeter intended for medical use and available without a prescription. Patients place a finger inside the device to measure their functional oxygen saturation of arterial hemoglobin and pulse rate. The device includes Bluetooth technology for wireless transfer of patient data to the Masimo Personal Health app.
February 4, 2024
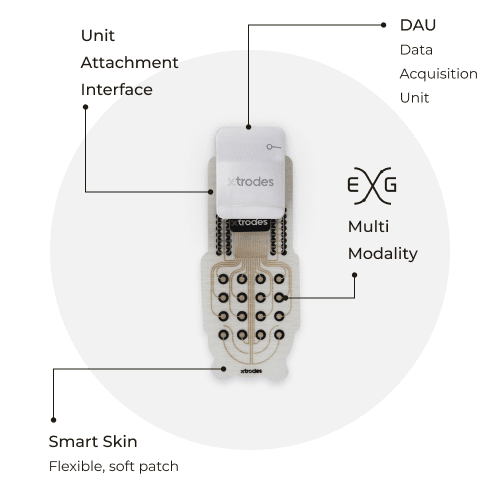
Source: X-trodes
The X-trodes System M is a connected medical device system that monitors and records a variety of physiological signals which are then sent via Bluetooth to a cloud server. The device consists of flexible patches known as Smart Skin that when worn can acquire medical-grade data while a patient goes about their day. Among the signals it records are electroencephalograms (EEG), electrocardiograms (ECG) and electromyograms (EMG).
February 15th, 2024

Source: Downloadable PDF on Neuro Spinal Innovation site
The SONIK MONARK 100 from Neuro Spinal Innovation is a medical device that aids in the management of chronic back pain and encourages spinal realignment. The device delivers controlled, low-frequency impulses through a stylus tip to the affected site. Treatment data is sent over Bluetooth to a connected computer.
February 15th, 2024

Source: Empatica
EpiMonitor by Empatica is a medical device system for monitoring seizures, consisting of a wearable EmbracePlus device and a companion EpiMonitor smartphone app. The wearable device senses electrodermal activity and motion data and detects potential tonic-clonic seizures in patients with or at risk of having epilepsy. If a seizure event is detected, the wearable device sends a notification over Bluetooth to the smartphone app, alerting the patient and their caregivers. Data collected by the device is also shared from the app with a cloud database.
March 5th, 2024
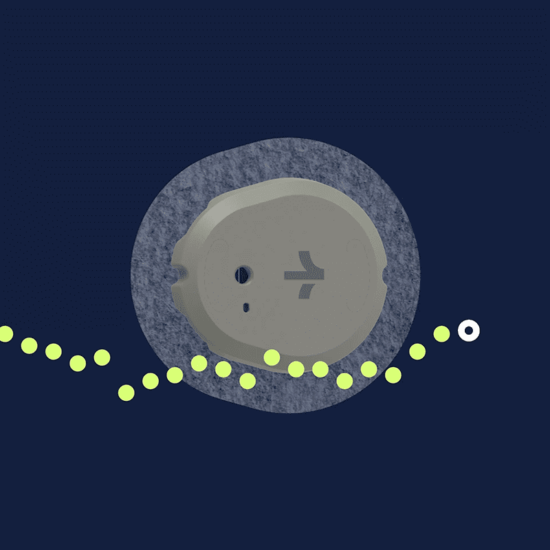
Source: Dexcom
Stelo is an integrated Continuous Glucose Monitor (CGM) available over the counter to patients with Type 2 diabetes who are not on insulin. Like other CGMs, Stelo continuously measures, analyzes and records glucose levels, as well as shares data over Bluetooth to a companion smartphone app. Unlike other CGMs, including Dexcom’s G7 platform, Stelo is not interoperable with insulin pumps, nor does it have the ability to suggest insulin dosages, as the device is intended for patients not on insulin.

March 15th, 2024
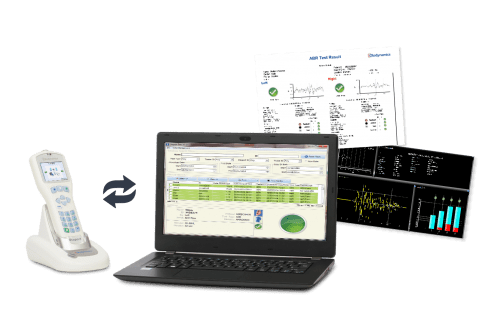
Source: Otodynamics
Otoport Advance is a handheld device used to non-invasively diagnose hearing loss in children and adults via Transient Evoked Otoacoustic Emissions (TEOAE) and Distortion Product Otoacoustic Emissions (DPOAE). Data from the Otoport Advance can be transmitted over Bluetooth to Otodynamics’s Otolink PC patient data management software suite.
April 10th, 2024
Source: Lapsi Health
The Keikku Electronic Stethoscope from Lapsi Health amplifies, filters and transmits listening data of a patient’s heart, lungs, bowels, arteries and veins to a companion smartphone app over Bluetooth. The Keikku app provides data annotation, visualization and audio playback, as well as sharing features so that the device can remotely monitor patients and serve as an input in telemedicine. The device is intended for use both in clinical settings and at home by patients.
April 19th, 2024

Source: Edan
The iX Series of patient monitors from Edan Instruments perform long-term continuous monitoring of a patient’s physiological parameters, including respiration rate, temperature, oxygen saturation, blood pressure and more. The device can transmit patient data over Bluetooth to Edan’s e-Link platform.
April 29th, 2024

Source: Transtek
The Transtek Blood Pressure Monitor measures systolic pressure, diastolic pressure and pulse rate using an inflatable cuff wrapped around the upper arm. Readings from the device sync over Bluetooth with the Transtek Health app, as well as a cloud database.
May 28th, 2024

Source: Clario
The Clario SpiroSphere is a compact spirometry device used to measure a patient’s inspiratory and expiratory lung function. With this 2024 filing, the device is now able to take 12-lead ECGs by connecting via Bluetooth to a compatible portable ECG device, the Corscience COR12.
May 29th, 2024
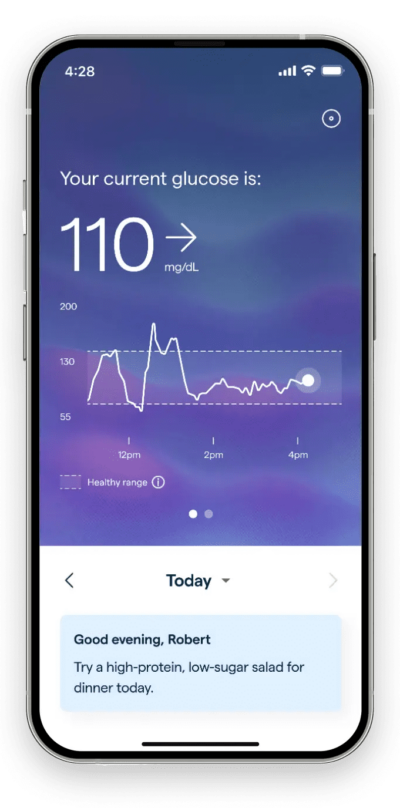
Source: Abbott
Abbott’s Lingo is an integrated CGM available over the counter, designed to monitor blood glucose levels of patients with Type 2 diabetes who do not use insulin. This device appears to compete directly with the Dexcom Stelo, as they share the same indication for use. The Lingo sensor worn on the body connects over bluetooth to a companion Lingo app, allowing patients to view their real-time glucose values, trends and graphs.
_________________________________________________________________________
Looking for a partner to develop your Bluetooth-enabled medical device? Orthogonal’s extensive library of edge cases, testing methods and mitigations will help you get the most out of Bluetooth communication for connected medical devices.
Related Posts

Article
Help Us Build an Authoritative List of SaMD Cleared by the FDA

Article
SaMD Cleared by the FDA: The Ultimate Running List

Article
Roundup: Bluetooth Medical Devices Cleared by FDA in 2023

White Paper
Software as a Medical Device (SaMD): What It Is & Why It Matters