
Article
Roundup: Bluetooth Medical Devices Cleared by FDA in 2024
Bluetooth 5.0 introduces a number of upgrades in speed, security and range to previous versions of Bluetooth. Yet many consumers still don’t have Bluetooth 5.0 compatible smartphones. In this article, we take a closer look at Bluetooth 5.0 support across today’s most popular mobile devices (Apple and Android), and offer those in the medical device industry guidance on when it makes sense to support Bluetooth 5.0 for your connected medical devices.
With over eight in 10 adults now owning a smart device, the “internet of everything” (IoE) has become more accessible to both patients and healthcare providers alike. In this new space, wireless connectivity such as Bluetooth is powering the next-generation of IoE technologies — which, among its many use cases, has enabled patients to receive care via their smartphones.
Two versions of Bluetooth are now available for Apple and Android devices: Bluetooth 4.2 and Bluetooth 5.0. Currently, the majority of devices with Bluetooth-connected mobile apps utilize Bluetooth 4.2; however, Bluetooth 5.0 offers several notable improvements over its predecessor and is rapidly gaining market share.
While it’s easy to take a “newer is better” approach in your development strategy, determining which version of Bluetooth to support requires a nuanced understanding of smartphone market share, user preferences, and upcoming smartphone trends: if your support goes too far back, you risk losing key Bluetooth benefits; conversely, developing too far into the future means you risk losing important market share.
In this article, we take a closer look at Bluetooth 5.0 support across today’s most popular mobile devices (Apple and Android), and offer those in the medical device industry guidance on when it makes sense to support Bluetooth 5.0 for your connected medical device(s).
Launched in December 2014, Bluetooth 4.2 introduced the IPv6 protocol, the most recent version of the Internet Protocol, for direct internet connection.
Version 4.2 supports both Bluetooth Classic and Bluetooth Low Energy (BLE). Bluetooth Classic is used to connect mobile phones to Bluetooth headsets for phone calls and for streaming applications such as audio streaming and file transfers. By contrast, BLE is a low power version of Bluetooth, meant for power sensors and accessories. Like Bluetooth Classic, BLE allows devices to communicate with one another, but is meant for a wider range of internet-enabled devices.
BLE was not meant to replace Bluetooth Classic, but rather, to serve as a way for Bluetooth devices to communicate quickly and easily with connected devices in the ever-growing Internet of Things.
The main benefit of BLE is that, as a low-power technology, it can communicate with nearby devices that must, by design, consume less power. These devices include wearables such as heart rate monitors and fitness devices, which typically must run on batteries for long periods of time and therefore cannot use a lot of energy.
BLE achieves its ultra-low power consumption by “turning the radio off” as much as possible, and sending small amounts of data at slower transfer speeds. Compared to Bluetooth Classic, BLE connections are also much faster, as discovery occurs on only three channels, compared to Bluetooth Classic’s time- and power-consuming 32-channel discovery process.
Indeed, the efficient and low-power nature of BLE, not to mention its ubiquity in most smartphones on the market and low-cost BLE modules and chipsets, makes it indispensable for connected medical device applications.
Bluetooth 5.0 was introduced in July 2016, and is currently the latest version of the short-range wireless connectivity standard. The main goal of Bluetooth 5.0 is to make Bluetooth compatible with more devices across the Internet of Things. Official specifications for Bluetooth 5.0 state that “Bluetooth 5 brings some major advances to [Bluetooth Low Energy] and makes it ideal for an even broader range of IoT scenarios … [Bluetooth 5.0] will have a substantial impact in many sectors and further position it as the low power wireless technology of choice in the Internet of Things.”
Bluetooth 5.0 offers a number of improvements on Bluetooth 4.2, and in particular, major updates to BLE:
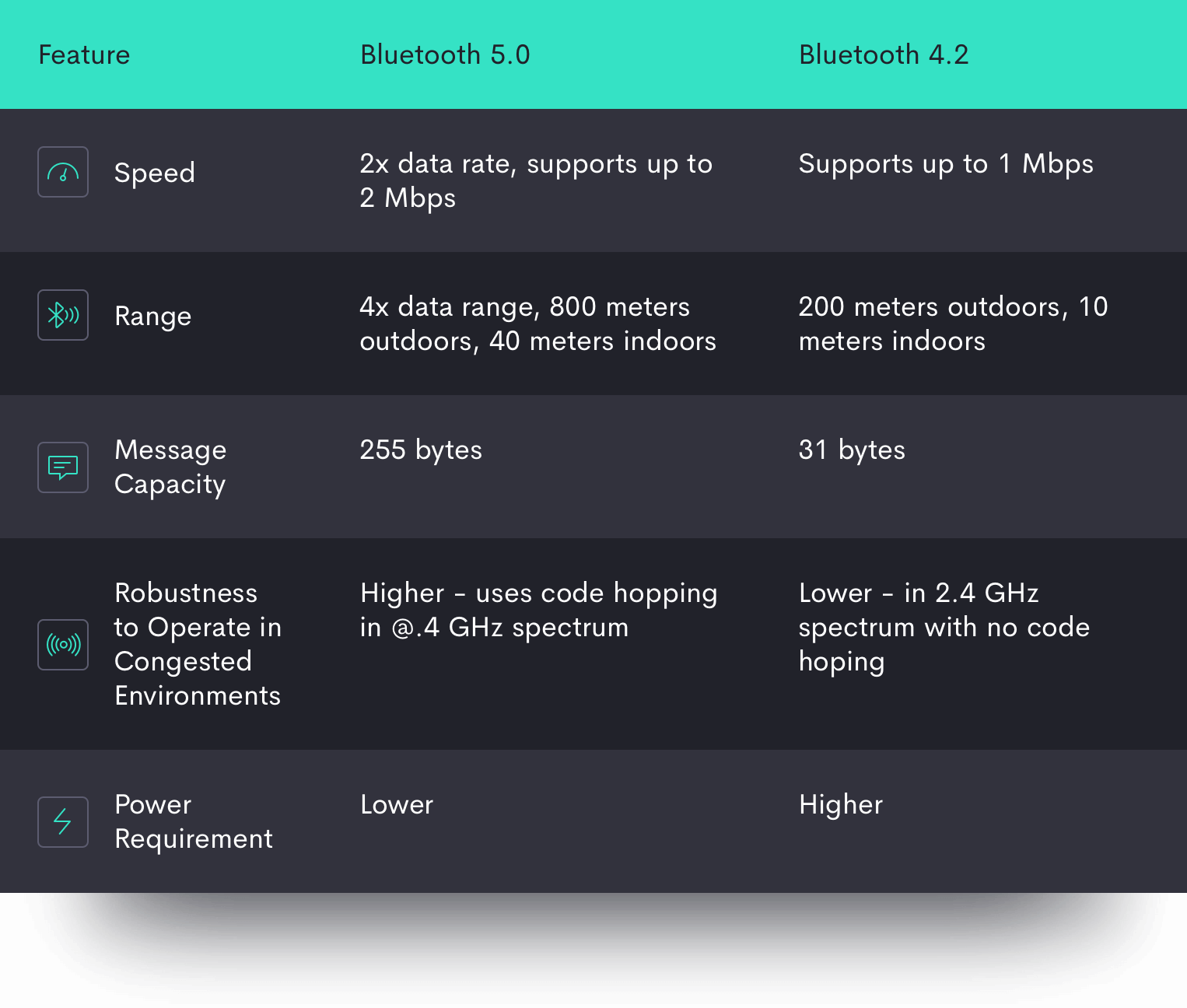
It’s important to highlight that not all smartphone models and OS variants that support Bluetooth 5.0 include all of the benefits of the new update, particularly on the Android side. These improvements are optional to varying degrees, so hardware manufacturers that support 5.0 may be missing one or more of these new features.
One important new feature in the Bluetooth 5.0 specification is a choice of 3 Physical Layers, or PHYs, which configures the physical parameters of the radiofrequency communications between devices; in essence, the PHY is the Bluetooth chipset. To run Bluetooth 5.0, the phone’s PHY must, at minimum, support LE 1M PHY, the chipset used in Bluetooth 4.0. However, there are two more advanced types of PHYs with better capabilities which do not have to be used in Bluetooth 5.0 smartphones.
The second type of PHY, the LE 2M PHY, is faster, and the LE Coded PHY provides about four times the range of Bluetooth 4.2 without increasing power usage. However, while most new smartphones on the market support either LE 2M PHY and LE Coded PHY, most do not support both.
A second new feature in Bluetooth 5.0 is the use of longer “advertising” packets. In this case, advertising refers to Bluetooth’s ability to broadcast its signal to identify itself to other Bluetooth devices. So-called Bluetooth “advertising” is essential so that the device can locate and link up with other devices. With a longer advertising packet, more information can be transmitted between the connecting devices, which serves to improve the user experience.
However, longer advertising is not required for all Bluetooth 5.0 devices, nor is it supported by devices that only run Bluetooth 4.2. This means that even if newer Bluetooth 5.0 smartphones support longer advertising lengths, older smartphones running Bluetooth 4.2 will not be able to communicate with them, causing compatibility problems between different generations and hardware models/variants of smartphones.
In short, three of the features available in Bluetooth 5 (LE 2M PHY, LE Coded PHY, and Extended Advertising) do not actually have to be supported for the device to be classified as Bluetooth 5.0.
Consequently, when a smartphone manufacturer says its hardware model supports Bluetooth 5.0, there may be limitations that prevent it from interfacing with other devices which have support for all of the new features of Bluetooth 5.0.
Unfortunately, this also means that many devices classified as Bluetooth 5.0 will not actually have all Bluetooth 5.0 features. For example, the Samsung Galaxy S9+ only supports the PHY 2M Layer, while Galaxy S10+ supports all three. This is why it is important to determine whether a device has support for the new Bluetooth 5.0 features before designing a Bluetooth-enabled SaMD product. As noted above, many flagship smartphone models are missing some of the new Bluetooth 5.0 features.
Starting in Q1 2016, over 20% of iPhone hardware models supported Bluetooth 4.2. The iPhone 6 was the first Apple smartphone model to support Bluetooth 4.2, followed by the iPhone 7, 7 Plus, and SE. Q4 2017 saw the introduction of Bluetooth 5.0, which is supported by the newer iPhones, including the iPhone 8, 8 Plus, X, XR, XS, XS Max, 11, 11 Pro, 11 Pro Max, and the just-released iPhone 12 models.
In Q1 2021, 99.4% of Apple iPhone hardware among North America’s subscriber base supported Bluetooth 4.2 and up, while 72.88% of phones supported Bluetooth 5.0 or higher. This data indicates that the vast majority of iPhone models used in North America meet both Bluetooth 4.2 and 5.0 standards and that over 70% of iPhones are Bluetooth 5.0 compatible.
Apple’s iOS 14 launch in September 2020 offered new Bluetooth BLE functionality. The iOS 14 software introduced support for Bluetooth Basic Rate / Enhanced Data Rate (BR/EDR) devices. BR/EDR is generally used for short-range, continuous wireless connections, which is why it’s ideal for IoT devices, such as connected medical devices. iOS 14 also ushered in Apple’s emphasis on greater security and privacy. Furthermore, the changes in the new iOS made it easier for developers to use the Bluetooth Application Programming Interface (API).
However, with any new iOS upgrade, there would be issues. We have heard reports on some issues related to Bluetooth connectivity by users when they update their iPhones to iOS 14. This article takes a deeper into the issues that arise with iOS 14.
Samsung Galaxy S7 was the first of Samsung’s flagship line of Galaxy smartphones to offer Bluetooth 4.2. The S8 was the first of the company’s Galaxy line of smartphones, as well as one of the first phones on the market, to offer Bluetooth 5.0. However, Samsung has, to date, been inconsistent in the implementation of Bluetooth 5.0 specification options in their hardware models. The Samsung Galaxy S9+ only offers some of the three primary 5.0 specification options, while the S10+ offers all three.
In addition to lack of hardware support, several older versions of the Android operating system do not offer full software compatibility with Bluetooth 5.0. At the present moment, Android versions 8 and above currently support both Bluetooth 4.2 and Bluetooth 5.0 (although not necessarily across all three domains).
Given this information, when should you actually target Bluetooth 5.0 for your connected device solution?
There are two parts to answering this question:
For the first part, it’s worth noting that many applications don’t need the higher bandwidth, range, advertising, or more robust coexistence in a congested environment. For example, if you’re only transferring a small amount of data between a device and your smartphone when the application is in foreground mode (and close to the device), then you likely don’t need Bluetooth 5.0.
If, on the other hand, you frequently transfer large amounts of data (like waveform or image data) when the smartphone application is in background mode and/or the smartphone is far from the device, or when you are controlling a medical device via BLE from your smartphone in a congested environment, then Bluetooth 5.0 improvements are much more useful—and in some cases necessary—to make your connected device system safe and feasible.
For the second part, a simplified answer is that it depends on when your next product release is scheduled and what level of market coverage you’re looking for.
According to ScientiaMobile’s MOVR report, as of Q1 2021, Bluetooth 5.0 covers approximately 72% of the iOS market share and Bluetooth 4.2 and earlier covers approximately 27% of the iOS market share. We expect Bluetooth 5.0 to comprise of 95% of the iPhone market by 2023.
On the Android side, the most popular Bluetooth version is also the 5.0 which covers approximately 56%, followed by Bluetooth 4.2 which covers about 24% of Android market. From 2018 to 2021, there was an increase of Bluetooth 5.0 from 18% to 56% among Android devices. On average, there is a 3% per quarter increase in Bluetooth 5.0 support on the Android side, as older models get replaced with the newer ones. We expect Bluetooth 5.0 to comprise of 95% of the Android market by 2023.
Ultimately, your strategy should take into account your need for Bluetooth 5.0 improvements, and when, where, and how much market share you need for the software you’re releasing. The recent data shows that, at least in the near term (e.g., Q4 2022), developing for Bluetooth 5.0 should be a top priority as its market share will only continue to increase.
For more insight into the smartphone trends affecting your connected medical device or SaMD products, download the latest version of our white paper: Smartphone Market Analysis for Connected Medical Devices & SaMD: Understand key trends and capture more market share with the right BYOD strategy.
Related Posts

Article
Roundup: Bluetooth Medical Devices Cleared by FDA in 2024

Article
Help Us Build an Authoritative List of SaMD Cleared by the FDA

Article
SaMD Cleared by the FDA: The Ultimate Running List

Article
Roundup: Bluetooth Medical Devices Cleared by FDA in 2023