
Article
Roundup: Bluetooth Medical Devices Cleared by FDA in 2024
This is the fourth and final blog in our late spring/early summer series titled “Sinking, Swimming, or Surfing: COVID-19 and The Great Acceleration of Digital Health.” In this article, we discuss four critical success factors for digital health solutions.
We conclude this blog series with a bit of background about Orthogonal and why we get up every day so excited to move the needle on healthcare by applying the power of fast feedback loops to connected mobile medical devices (CMMD) and Software As a Medical Device (SaMD).
**
The tragedy of COVID-19 has opened up a rare window of opportunity for innovators and leaders to have a considerable impact on healthcare costs and outcomes. In this blog series, we have shared advice from what we see in the market for how firms can take advantage of this window to make huge gains with great digital health solutions, including connected mobile medical devices (CMMD) and Software as a Medical Device (SaMD).
In the first blog in this series, we explain why major investors in the markets have signaled that they see this as a time for MedTech companies to make thoughtful, strategic, and possibly significant moves to position themselves for what some are now calling “the COVID-19 economy.”
In the second blog, we explain how the emergency responses to COVID-19 has dramatically accelerated seven trends that were already underway in healthcare generally and have created a window of opportunity for digital health to step in to solve major issues that now have more urgency.
In the third blog, we look at how we expect digital health and the use of CMMD and SaMD to continue to accelerate and evolve after the COVID-19 pandemic has passed.
In this final blog, we now look at…
Digital health is still in relative infancy in terms of adoption and impact. However, there are key insights, best practices, and approaches that are already proven to mean the difference between impactful digital health solutions and seemingly “cool” solutions that flop because they ultimately fail to deliver two key things:
In other words, the key to developing successful CMMD and SaMD is to spend your time and money making new and exciting mistakes, not old and proven ones. There are three places we should look for these insights:
In the rest of this article, we look at four key, interrelated related success factors for digital health solutions, including CMMD and SaMD.
Compared to the physical world, which is bounded by the limitations of biology, chemistry, and physics, the digital world of software and data is far more open, malleable, flexible, agile, fluid, and any other relevant adjective you want to throw in here.
One of the biggest challenges when shifting from physical-only solutions to hybrid physical-digital solutions is not to constrain our thinking with an analog mentality. In other words, we can’t fall into the trap of only being able to see the possibilities of our old Flatland when digital has opened up new dimensions. In the world of digital health, we can begin to think in terms of solutions that combine multiple building blocks. We can think in terms of A and B, instead of A or B.
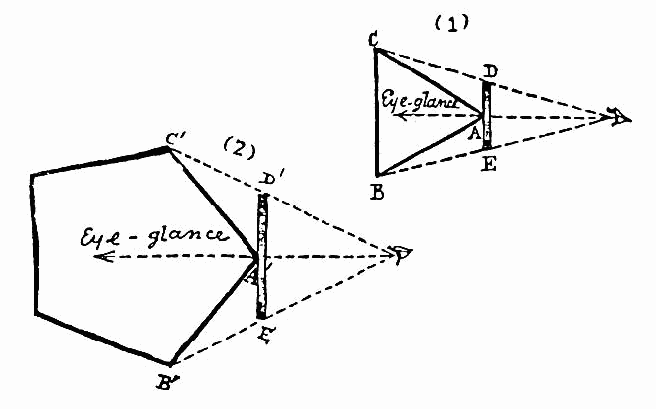

Black and white images from Flatland: A Romance of Many Dimensions (Illustrated) by Edwin Abbott. Color image by Valero Doval in Wired Magazine.
For digital health, CMMD, and SaMD, we suggest being open to thinking differently about three domains: solutions over products, physical location, and business models.
Solutions over Products
As we’ve noted before, healthcare dollars to continue to migrate from fee-for-service models, where care is priced based on what their providers do, to value-based models, where the cost of care is tied to outcomes. In other words, we are now paying people to solve health problems with measurable benefits instead of providing health products with quantifiable features.
There is a similar debate going on in higher education where universities and colleges charge students by the credit hour and require a certain number of credit hours to earn a degree. However, students don’t think of paying for college as multiple years of buying credits. They think of themselves as buying the total package of a college education and experience, which includes a combination of things that will happen during their studies and a different combination of things that this entire package will set them up for in life after their studies.
This trend is tailor-made for digital health, where there is considerable flexibility to stitch together multiple products in a way that achieves predefined outcomes. We have seen this trend in the growth of regulated combination products that blend drugs, biologics, and devices.
The Flexibility of Multiple Locations
Digital health enables more sophisticated combinations of care coordination by stitching together teams of people in different places, working at different times on various tasks. As a result, healthcare solutions will continue the trend of being less-centered on a single location such as a hospital or doctor’s office.
For example, a medical team charged with monitoring patients with a chronic disease will be able to communicate with a patient at the exact right moments in time with the minimum necessary inconvenience for everyone involved. Using sophisticated analytics (and sometimes just descriptive analytics), care teams can use connected solutions to monitor their patients continually, and only have to communicate with the patient at the moment when there is an emerging concern, or it is the prescheduled time to reassess treatment. When those communications happen, patients may need to come to a testing center, clinic, or hospital. But they might also simply have a phone call or video chat with a doctor, possibly augmented by mailing in a test sample or having a visiting RN come to the house to do a hands-on evaluation or administration of a more compact treatment.
Alternative Business Models
Digital health solutions lend themselves a wider variety of business than more traditional business models, such as the industry model from consumer packaged goods of manufacturer/distributor/retailer or the McDonald’s model of franchisor/franchisee.
The book Business Model Generation and its framework have had a significant impact on our collective understanding of these options. It is much easier to consider alternative models using tools like the Business Model Canvas and their directory of business models that are easier to implement in a digital world, such as Unbundling, The Long Tail, Multi-Sided Platforms and Freemium.
Picture 1: Blank Business Model Canvas by Business Model Alchemist, CC BY-SA 1.0. Picture 2: by JAM Visual Thinking, Amsterdam. Picture 3: Colorful filled out canvas.
While these alternative business models could have a significant impact on healthcare and MedTech, health economics still has more in common with the scheming, warring factions, and shifting alliances of Game of Thrones than it does with supply and demand, invisible hand, and rational choice concepts of economics. So, as with real-world data (RWD) and real-world evidence (RWE), we need to temper our expectations about the potential of flexible new business models. Tremendous constraints remain in healthcare, many of which have been loosened by COVID-19, but none of which are simply going to vanish. These are the kinds of questions to ask when you are considering new, digitally-enabled business models:
Thinking differently about the domains of solutions over products, physical location, and business models has the potential to reduce the burden on overworked doctors, nurses, and other clinicians, lower the cost of care, and improve outcomes.
As we have learned from the behavioral sciences, having more options is not necessarily better. The human brain is not innately wired to help us make the best choice when the possibilities seem limitless.
For anyone who wants to see this concept in action, I recommend taking a child you love and adore, handing him or her a $10 or $20 bill, and walking into the largest candy store in America, b.a. Sweetie Candy Company in Cleveland. Once the initial shock and awe of this fantastic store wears off, you are likely watching a child who is either suffering from analysis paralysis or realizing that $10 or $20 doesn’t even begin to scratch the surface of what they wish to buy.
Is this the greatest candy store ever?
Since digital health, CMMD, and SaMD create so many options, the ultimate winners are often the firms with a rapid and consistent process for defining overall goals and success criteria and then narrowing down the multitude of options into the best few candidates. Then the winning firms methodically navigate their way to a successful launch of a solution and follow up that launch with a continuous stream of rapid updates that steadily increase its value.
While we are always hesitant to proclaim any one way of thinking as a panacea, after spending two decades in the digital product development business (with an exclusive focus on CMMD and SaMD for the last decade), we do believe that fast feedback loops (FFLs) and many different product development methods built on them are at the core of what separates digital health surfers from digital health swimmers and sinkers.
FFLs are the process by which we take large, complex product development pipelines and break them down into a repeatable stream of highly effective build-measure-learn cycles. In other words, what we are doing is combining the following basic principles into a powerful tool for consistently arriving at success:
It is the application of fast feedback loop techniques to the new digital technologies that led to new expressions like “moving at web speed” and “moving at internet speed” as updates versions of Star Trek’s “moving at warp speed.”


Sources for Star Trek graphic and traffic light photo.
These are some highlights of what it will feel like for firms that embrace this approach to MedTech product development:
Shifting to fast feedback loops is not an all-or-nothing proposition. It is an evolution that—in the spirit of its legendary ancestors, Edward Deming and John Boyd—calls for a teamwork culture and related processes that embrace continuous quality improvement as a virtuous cycle in and of itself.

Put simply; you can’t mandate that patients and clinicians actively engage with digital health solutions. At most, you might be able to force them to go through the motions of performing specific actions with a tool.
For example, Electronic health record (EHR) systems should empower doctors to work faster, better, and smarter. So ask yourself, how many times have you had a clinic visit with a doctor where you watched the doctor struggle against their EHR system to get their job done? Now ask yourself, how many times have you seen your doctor effortlessly commune with their EHR system, using it as a powerful extension of their skills and knowledge in the same way that a journalist or author does with their keyboard and screen? Or have you ever heard a doctor say, “You can have my EHR system when you pry it from my cold, dead fingers?”
Moving the needle on healthcare costs and outcomes requires everyone involved to be motivated to help the solution succeed. It all boils down to empowering patients and clinicians to take new approaches to health management by understanding their worlds, solving their problems, and providing benefits to them that are meaningful. These are the solutions that gain adoption, drive value for the stakeholders, and grow successful businesses.
This pattern has repeated in recent decades in healthcare[2] as well as in many other industries and sectors. It’s especially the case with digital health, where patients, caregivers, and clinicians (e.g., doctors, nurses, pharmacists, and physical therapists) expect their digital health solutions to be as convenient, useful and satisfying as the rest of their digital experiences. And without patient engagement, digital health is either an unused tool, a mandatory reporting system, or a fusion of glitz and vanity metrics that sound great but have little substance.
To get good user engagement with your digital health solution, you need to put the time to observe, communicate with, and truly understand what motivates patients and healthcare providers, as well as gain a deep understanding of the barriers to initial adoption and continued engagement.
The good news is that there is a well-developed and proven suite of qualitative and quantitative methods and tools that support this hard work. These methods include user research, identification and testing of patterns, and user feedback on designs and prototypes. They also include tracking how your users are behaving and how your interventions are affecting that behavior through person-based analytics. It’s important to note that while these are all potentially powerful techniques, their real power is unleashed when paired with iterative, fast feedback loops.
One of the advantages of our newly connected world is that today it is orders of magnitude cheaper and easier to acquire, store, and effectively analyze vast volumes of data that instrument and quantify what happens in the real world over time. This data abundance creates an opportunity to augment (but not replace) the current data sources of R&D and clinical trials.
Those traditional data sources are invaluable because their scientific quality gives us confidence in our MedTech products. However, these conventional types of data are slow and expensive to collect. As a result, we carefully design the scope and frequency of data collection to balance tradeoffs in budget and schedule and collect statistically valid data.
Some critical sources of real-world data that are relevant to digital health include the following:
There is enormous potential for RWD and RWE, combined with a healthy dose of creative thinking and fast feedback loops, to transform virtually every phase of the MedTech product life cycle including:
To the extent that digital health product makers demonstrate the validity of their data to regulators and payers, it can be a massive accelerator to their decisions to approve these devices as being safe and clinically effective enough to enter the market. Also, these data can demonstrate the comparative effectiveness of these products, which impacts not just the yes or no decision about whether to provide coverage for this solution, but also how much it’s worth paying (i.e., reimbursing) for this solution.
For MedTech firms, that’s an incredibly powerful trifecta:
Orthogonal is a software developer for connected mobile medical devices (CMMD) and Software as a Medical Device (SaMD).
We work with change agents who are responsible for digital transformation at medical device and diagnostics manufacturers. These leaders need to accelerate their pipeline of product innovation to modernize patient care and gain a competitive advantage.
Orthogonal applies deep experience in CMMD/SaMD and the power of fast feedback loops to rapidly develop, successfully launch, and continuously improve connected, compliant products—and we collaborate with our clients to build their own rapid CMMD/SAMD development engines.
Almost twenty years ago, we began our consulting work to create great software products in a range of industries, including financial services, education, research, and healthcare. We spent nearly a decade working with leading-edge FFL techniques that have now become recognized best practices such as Agile software development (with XP, Scrum, and Kanban), Lean User Experience (Lean UX), and Lean Startup.
Nearly a decade ago, we got a glimpse of digital healthcare and the enormous potential of the cloud, IoT, and smartphones, and the potential impact of medical device software. Realizing that’s what we wanted to focus our energies on exclusively, we narrowed our focus to the development of CMMD and SaMD. We’ve never looked back.
Our team gets up every day excited to help move the needle on healthcare. For almost ten years, we’ve helped a wide variety of firms develop and bring their regulated/connected devices to market. We’re even more excited about the next ten years and what we will help accomplish during The Great Acceleration of Digital Health.
If you need help building your next CMMD or SaMD, or to learn more, contact us or call us at (866) 882-7215.
We will continue to dive into the best of what we are reading and hearing about in the industry. Some of the topics we plan to address include:
Bernhard Kappe is Founder and CEO at Orthogonal. You can email him at bkappe@devorthogonal.wpengine.com.
Randy Horton is VP of Solutions and Partnerships at Orthogonal. You can email him at rhorton@devorthogonal.wpengine.com.
Footnotes:
Related Posts

Article
Roundup: Bluetooth Medical Devices Cleared by FDA in 2024

Article
Help Us Build an Authoritative List of SaMD Cleared by the FDA

Article
SaMD Cleared by the FDA: The Ultimate Running List

Article
Roundup: Bluetooth Medical Devices Cleared by FDA in 2023