
Article
Top 10 SaMD Job Boards & Regional Organizations
This blog contains Chapter 5 of the Orthogonal eBook titled: Digital Therapeutics (DTx): Accelerating Success Using Fast Feedback Loops. The following are links to each chapter of this eBook:
Because a DTx is, by definition, digital, data creation is a natural byproduct of the therapeutic process. Data collection is a marginal cost and low-effort activity compared to data collection during traditional clinical trials or post-market surveillance.
This is true not only for DTx, but for most types of connected software and connected IoT devices. IoT has made it much easier to collect timely performance data about the operation of the software or physical device, as well as other ambient data that is readily available at the point-of-usage. These data can be used to provide real-time, evolving insights into the usage of the product and the relevant lives of the product user base at both an individual level as well as in aggregate across the entire user base. In turn, these data can be used to inform future product changes implemented in a process of continuous iteration, improvement, and optimization.
Data streams generated by DTx collect useful data that can be leveraged in many different contexts and at a variety of points along the product life cycle. For example, although the ability to collect more and better data during clinical trials is a clear benefit in MedTech, the same data collection process can continue when the product is being used in the real world. Real World Data (RWD) and Real-World Evidence (RWE) are becoming increasingly important across the digital health ecosystem. The FDA’s definitions of these two terms:[1]
The value of RWD and RWE become clear in consideration of the controlled environment of a clinical trial and the limitations of extrapolating trial results to the real world. Clinical trials have small sample sizes compared to the general population and patients are on regimented and monitored dosing cycles with several scheduled check-ins. This scenario is unlike the real world in which patients are expected to self-manage medication adherence without frequent check-ins with their clinical team.
Monitoring and analyzing RWD allows a more comprehensive understanding of therapeutic efficacy and adverse effects in different populations. For traditional drug-based therapies, variance of certain genes (i.e., genetic polymorphisms) can impact drug metabolism. This leads to different dosing requirements based on genetic predispositions. An excellent example is the CYP450 gene,[3] which metabolizes 75–90% of all drugs, including beta blockers and antidepressants, and has over 2,000 mutations.[4]
For DTx, genetic mutations on drug metabolizer genes are less important, but analyzing RWD on patient adherence and engagement with DTx can help identify areas of opportunity to improve the product. The data-rich nature of DTx in combination with drug therapy is appealing to the pharmaceutical industry because data can be a tremendous competitive advantage in the pursuit of high-quality data that can lead to and prove both the clinical and comparative effectiveness of different therapeutic approaches.
There are limitations in DTx clinical trials. Clinical trial participants who self-select into a DTx trial might have a predisposition to enjoying or being more engaged with digital interventions. Also, when participating in a relatively short-term trial with a research team that schedules several check-ins, participants might be more inclined to remain engaged and adherent than the average patient. These potential participant biases should be considered when evaluating DTx trial outcomes. That is why data created when the DTx is “in the wild” and patients are using the DTx in their day-to-day lives outside of a controlled trial can provide a more accurate picture of patient adherence and, ultimately, DTx efficacy.
Under these circumstances, DTx is uniquely positioned to be successful in delivering improved outcomes. When fast feedback loops are established correctly, as RWD/RWE becomes available, teams can pinpoint areas of opportunities for improvement and iterate on the product to address the needs of the users. Unlike traditional pharmaceuticals, DTx can continually monitor the patient population and remain agile in learning from that data, rapidly adjusting course and then repeating the analytic cycle.
For example, combination products that blend DTx and traditional pharmaceuticals, where a manufacturer is collecting more comprehensive data about their drugs as a byproduct of the data generated by DTx, can create a compounding competitive edge over companies that don’t have this class of data. In addition, since DTx are typically drug agnostic, companies that adopt this complementary approach have the potential to collect large amounts of actionable data far beyond their own patient populations.
RWD can be used to make changes to the DTx and how it is working to optimize or improve real-world performance at a relatively fast pace. The ease of access to RWD provided by DTx becomes even more attractive when you consider pushing updates to the therapy. Unlike conventional drug therapies, many updates to a DTx carry little patient risk and could potentially be applied on a frequent basis (see Section 6: Regulatory Approaches for DTx Manufacturers). This kind of data-driven frequent update can be especially powerful when it is integrated with AI/ML algorithms that are capable of continuous learning and evolution.
Product Analytics is a powerful class of software and data that is widely used across many tech-enabled industries. A relative newcomer in the overlapping product spaces of SaMD, connected medical devices, and DTx, product analytics are tools and techniques that collect an integrated set of data about user behaviors that allows analysis at the individual user level and in aggregate to gain insights about larger patterns and trends. The value of product analytics is broadly recognized and captured in domains such as online subscription services, social media and e-commerce, and it is key to keeping apps and solutions continually relevant and valuable to their users.
When applied to DTx, product analytics has three broad uses:
Although the use of product analytics for DTx and other types of medical devices is relatively nascent, it is a well-established domain with a mature set of commercial software products such as Mixpanel (which is Orthogonal’s preferred solution) and supporting analytic techniques. One example of this is A/B testing,[6] which helps determine the best-performing version of an app, product feature, or webpage. Adapted to DTx, an A/B testing methodology can be applied to determine which feature, or set of features, generates the most user engagement or adherence for certain patient cohorts, as well as the best health outcomes. Individualized patient engagement tactics can be applied based on patient behaviors.
Obviously, adapting the tools and techniques that big tech companies such as Facebook and Amazon use to make their products and services compelling raises questions related to privacy, safety, ethics, and compliance. For example:
As the DTx space evolves, we expect that MedTech will collectively develop the answers to these questions and similar ones that will undoubtably arise.
For management of chronic conditions such as COPD, diabetes, and hypertension, behavior changes can often have the greatest long-term impacts, particularly when coupled with more traditional treatments. According to a 2018 study in the Annals of Pharmacotherapy, medication non-adherence is one factor that is driving prescription drug-related mortality, which in total costs the U.S. an estimated $495.3 billion to $672.7 billion annually, and results in an estimated 275,689 deaths annually.[7]
DTx presents an opportunity to reduce some of these material and human costs because these products are designed to be in the hands of patients. Digital patient engagement tactics can be embedded into the DTx, allowing people to take control of their health by boosting their engagement and playing a greater role in the prevention, management, and treatment of their disease or condition. Various patient engagement features such as goal setting, symptom tracking, personalized medication adherence reminders, tailored education, and gamification can be implemented to digitally engage the patient. Evidence has shown that patient engagement can improve outcomes for smoking cessation, alcohol consumption, weight management, and self-management of chronic conditions.[8]
There are two identified limitations to patient engagement data that can be solved over time as this domain continues to gain traction. The first limitation to these data is the overall lack of RWD. Clinical trial data are currently available only for the small number of DTx that have completed. The second limitation is the current relative lack of evidence regarding the effectiveness of different patient engagement techniques.
Gamification provides a useful example of both of these limitations. There is promising evidence that, in a trial setting, gamification can result in positive health outcomes.[9] However, gamification seems to show diminishing returns once the novelty of the intervention wears off. In response, Villar[10] suggests that more individualized approaches to gamification and tailoring content to patient interest may be successful. Additionally, a study by Michie published in the Journal of Medical Internet Research highlighted the importance of analyzing user engagement with RWD to stratify patients and personalize engagement tactics that work best for each cohort.[11] Interestingly, both of these suggested approaches rest on an ability to more easily collect product analytics data from DTx usage.
An excellent distillation of the clinical value digital tools can add to patient care appeared in a 2020 npj Digital Medicine article. Reproduced in Figure 4 below, Rowland et al. defined categories of digital tools and the clinical scenarios where digital tools can offer value.[12] Building on this valuable theoretical construct, Orthogonal foresees that DTx can create a platform that can be extended to also support the other three categories. That is, DTx can provide therapy along with patient-reported outcomes (e.g., symptom-tracking surveys), behavioral change interventions (e.g., personalized medication reminders), and disease-related education.
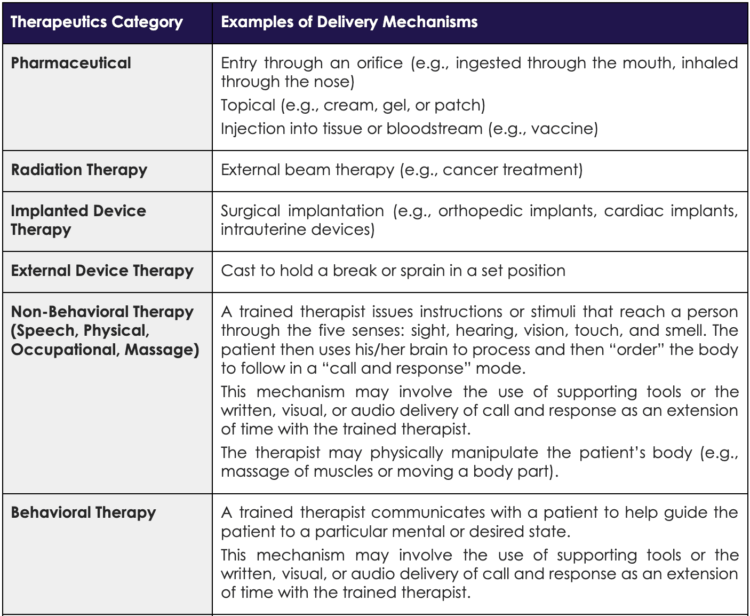
Figure 4. Figure from Rowland et al. paper: “What is the clinical value of mHealth for patients?”[13]
Finally, as with any prescription Tx, clinicians are the gatekeepers to and drivers of DTx adoption by patients (as discussed in Adoption in Section 8). Clinicians rely on evidence-based scientific research to determine effectiveness for patient care. The more clinical evidence that emerges around the impact of digital patient engagement, the more data and evidence the DTx industry will have to support clinician and patient use of this new class of Tx.
The symbiotic relationship between DTx and data are illustrated by the following two case studies (DarioHealth and BlueStar).
DTx offered alongside drugs and treatment pathways can boost patient engagement, enhance therapy adherence, and improve health outcomes.
For example, DTx can impact the significant population health consequences of chronic diseases such as diabetes. According to a report by Centers for Disease Control and Prevention (CDC) in 2020,[14] people living with diabetes represent 10% of the U.S. population, and diabetes care carries a multibillion-dollar financial burden in the U.S. alone.
Founded in 2011, DarioHealth delivers a diabetes disease management platform hyper-focused on personalized patient engagement that results in sustained behavior change. The DarioHealth type II diabetes patient engagement platform was used to study the effects of personalized patient engagement.[15] In the study, DarioHealth focused on “a powerful combination of AI, dynamically personalized user journeys, coaching, and behavior science that drives deeper, more meaningful engagement to generate better outcomes.”[16] DarioHealth’s Chief Medical Officer, Omar Manejwala discusses the power of personalized engagement using DTx in his article “Digital Health Beyond The Nudge.”[17]
Another DTx tackling diabetes management is WellDoc’s BlueStar®, which has garnered FDA approval as an evidence-based patient self-management tool.[18] With BlueStar, patients and providers use real-time data and feedback to support healthy behaviors such as drug adherence, diet and exercise control, and psychosocial wellness. BlueStar has demonstrated the capability to reduce A1C levels without any additional pharmacological interventions. This smartphone app can help chronically ill patients stay healthy without taking more drugs, demonstrating the potential to accrue economic benefits across the care spectrum.
BlueStar illustrates the idea of how persuasive approaches such as gamification that into account the motivational mechanisms in the human brain can to use them for the best health outcomes.[19] As the DTx market evolves, the industry will continue to evaluate the long-term, sustained impact of patient engagement tactics in the real-world to fully understand their applicability beyond clinical trials with small patient populations, shorter-term durations and artificially high levels of study controls.
Conclusion
The novel nature of a DTx offers many advantages compared to traditional pharmaceutical development, including cheaper production, faster time to market, and enhanced data collection. Further, when used as a complement to more traditional drug therapies, pharmaceutical companies can deliver better health outcomes while gaining a competitive edge in the marketplace.
This blog contains Chapter 5 of the Orthogonal eBook titled: Digital Therapeutics (DTx): Accelerating Success Using Fast Feedback Loops. The following are links to each chapter of this eBook:
References for Chapter 5 1. Real-World Evidence. U.S. Food and Drug Administration. https://www.fda.gov/science-research/science-and-research-special-topics/real-world-evidence. Published 2021. [Accessed August 23, 2021]. 2. U.S. Food and Drug Administration. 2021. Real-World Evidence. [online] Available at: <https://www.fda.gov/science-research/science-and-research-special-topics/real-world-evidence> Published 2021. [Accessed 19 August 2021]. 3. Goh L, Lim C, Sim W, Toh L, Leong K. Analysis of Genetic Variation in CYP450 Genes for Clinical Implementation. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5207784/. Published 2017. [Accessed August 23, 2021]. 4. T, L. and A, P., 2021. The effect of cytochrome P450 metabolism on drug response, interactions, and adverse effects. [online] PubMed. Available at: <https://pubmed.ncbi.nlm.nih.gov/17708140/> Published 2007. [Accessed 19 August 2021]. 5. Moll B, Yew K. White Paper: Accelerating SaMD and DTx with Product Analytics. Orthogonal. https://devorthogonal.wpengine.com/insights/accelerating-samd-and-dtx-with-product-analytics/ Published 2021. [Accessed August 24, 2021]. 6. Gallo A. A Refresher on A/B Testing. Harvard Business Review. https://hbr.org/2017/06/a-refresher-on-ab-testing. Published 2017. [Accessed August 23, 2021]. 7. Watanabe J, McInnis T, Hirsch J. Cost of Prescription Drug–Related Morbidity and Mortality. SAGE Journals. https://journals.sagepub.com/doi/10.1177/1060028018765159. Published 2018. Accessed December 6, 2021. 8. Perski, O., Blandford, A., West, R. and Michie, S., 2021. Conceptualising engagement with digital behaviour change interventions: a systematic review using principles from critical interpretive synthesis. [online] Available at: <https://doi.org/10.1007/s13142-016-0453-1> Published 2017. [Accessed 19 August 2021]. 9. Johnson, D., Deterding, S., Kuhn, K., Staneva, A., Stoyanov, S. and Hides, L., 2021. Gamification for health and wellbeing: A systematic review of the literature. [online] Available at: <https://www.sciencedirect.com/science/article/pii/S2214782916300380?via%3Dihub> Published 2016. [Accessed 19 August 2021]. 10. Villar, R. and Villar, R., 2021. One-size-fits-all gamification is not enough to improve patient outcomes. [online] Med-Tech Innovation | Latest news for the medical device industry. Available at: <https://www.med-technews.com/medtech-insights/one-size-fits-all-gamification-is-not-enough-to-improve-pati/> Published 2020. [Accessed 19 August 2021]. 11. Michie S, Yardley L, West R, Patrick K, Greaves F. Developing and Evaluating Digital Interventions to Promote Behavior Change in Health and Health Care: Recommendations Resulting From an International Workshop. https://pubmed.ncbi.nlm.nih.gov/28663162/. Published 2020. [Accessed August 19, 2021]. 12. Rowland S, Fitzgerald J, Holme T, Powell J, McGregor A. What is the clinical value of mHealth for patients? https://doi.org/10.1038/s41746-019-0206-x. Published 2020. [Accessed August 19, 2021]. 13. Rowland S, Fitzgerald J, Holme T, Powell J, McGregor A. What is the clinical value of mHealth for patients? https://www.nature.com/articles/s41746-019-0206-x. Published 2020. [Accessed August 23, 2021]. 14. National Diabetes Statistics Report, 2020 | CDC. Cdc.gov. https://www.cdc.gov/diabetes/data/statistics-report/index.html. Published 2020. [Accessed August 24, 2021]. 15. Fundoiano-Hershcovitz Y, Hirsch A, Dar S, Feniger E, Goldstein P. Role of Digital Engagement in Diabetes Care Beyond Measurement: Retrospective Cohort Study. https://pubmed.ncbi.nlm.nih.gov/33599618/. Published 2021. [Accessed August 19, 2021]. 16. Corp. D. DarioHealth Study Examines Connection Between Personalization and Sustained Behavior Change in Digital Health. Prnewswire.com. https://www.prnewswire.com/il/news-releases/dariohealth-study-examines-connection-between-personalization-and-sustained-behavior-change-in-digital-health-301238338.html. Published 2021. [Accessed August 19, 2021]. 17. Manejwala O. M.D. Digital Health Beyond the Nudge - DarioHealth. DarioHealth. https://www.dariohealth.com/articles/digital-health-beyond-the-nudge/. Published 2021. [Accessed August 19, 2021]. 18. Digital Health Platform Solutions | Welldoc. Welldoc | Chronic Care Platform. https://www.welldoc.com/chronic-care-management-platform/. Published 2021. [Accessed August 19, 2021]. 19. The Top 15 Examples of Gamification in Healthcare - The Medical Futurist. The Medical Futurist. https://medicalfuturist.com/top-examples-of-gamification-in-healthcare/. Published 2017. [Accessed August 19, 2021].
Related Posts

Article
Top 10 SaMD Job Boards & Regional Organizations

Article
Top 10 SaMD Events, Conferences & Webinars

Article
Top 10 SaMD Publications, Blogs & Media

Article
Top 10 SaMD Communities, Organizations & Working Groups