
Article
Roundup: Bluetooth Medical Devices Cleared by FDA in 2024
This is Part One of a two-part interview between Orthogonal’s Randy Horton and Danny Bernstein of metaMe Health.
Read Part Two of the interview here: metaMe’s Journey From Startup to FDA Clearance
Orthogonal’s Randy Horton, VP of Partnerships, sat down with long time partner, client and colleague Danny Bernstein from metaMe Health. Their Digital Therapeutics (DTx) app Regulora just received FDA clearance as a Prescription Digital Therapeutic (PDT). There’s a lot to learn from their years of work getting Regulora ready to market.
Regulora treats irritable bowel syndrome (IBS), a condition that affects 10% of people worldwide. Danny’s personal experience with IBS led him to the North Carolina protocol: an effective gut-directed hypnotherapy protocol for treatment of IBS, but whose distribution is limited to primarily major medical teaching centers in the U.S. because of the small number of practitioners trained in this highly specialized technique. Danny wondered if this therapy could be brought to a larger patient population through digital health.
Danny and the team at metaMe started in telehealth, but pivoted to PDT in order to scale the solution. Their app Regulora administers the protocol the same way every time. Its effectiveness is on par with in-person therapist treatments, and boasts a 90% adherence rate in clinical trials. Non-invasive, effective and with minimal side effects, it has the potential to vastly improve the lives of patients who are suffering from IBS.
In Part One of this interview, Danny gives an overview of IBS from his perspective as a patient, and describes the Regulora patient life cycle, from prescription to end of treatment.

Randy Horton:
Hey Danny, thanks for joining today. You guys just reached an incredible milestone at metaMe, which was your FDA 510K clearance for your prescription digital therapeutic (DTx). There’s fewer than 10 companies that have done that so far. That’s a real milestone.
We wanted to have you in today to talk a bit about the journey you’ve been on at metaMe, where you all started, what lessons you learned and what advice you have for other people in this space. Take it from the top and tell us a bit about metaMe and the problem you’re solving.
Give me a little background on you to start. I’m sure you weren’t in kindergarten thinking, “I’m going to make a digital therapeutic for irritable bowel syndrome.” How did you land in this venture and when did you land in this?
Danny Bernstein:
It’s actually kind of funny that you say that, because it’s not very far off. I come at this from a patient’s perspective. Since my early teens, I’ve been living with IBD Crohn’s[1] as well as IBS.[2] It’s a problem I’ve always lived with, and I’ve tried to find ways to improve my own situation.
On the IBD Crohn’s side, I managed to avoid flare-ups primarily through diet for many years, until I wasn’t, and then surgery and biologics became the plan. On the IBS side, I learned a lot from my GI doc, Dr. Stephen Hanauer at Northwestern Medicine Digestive Health Center. He was my introduction to behavioral medicine, like hypnotherapy known as the North Carolina protocol.[3] This was a way of treating patients with IBS that was very helpful, with many clinical studies that showed safety and effectiveness, but was not accessible to the vast majority of people. This began my learning on how to realize the possibilities and potential to make behavioral medicine for IBS a much more accessible therapeutic.
Randy Horton:
Can you give me a little background on IBS, its prevalence, what populations it hits and what the impact is that you’re trying to mitigate? Is it something that can be cured? Is it something that can be better managed? What’s the personal impact on a patient?
Danny Bernstein:
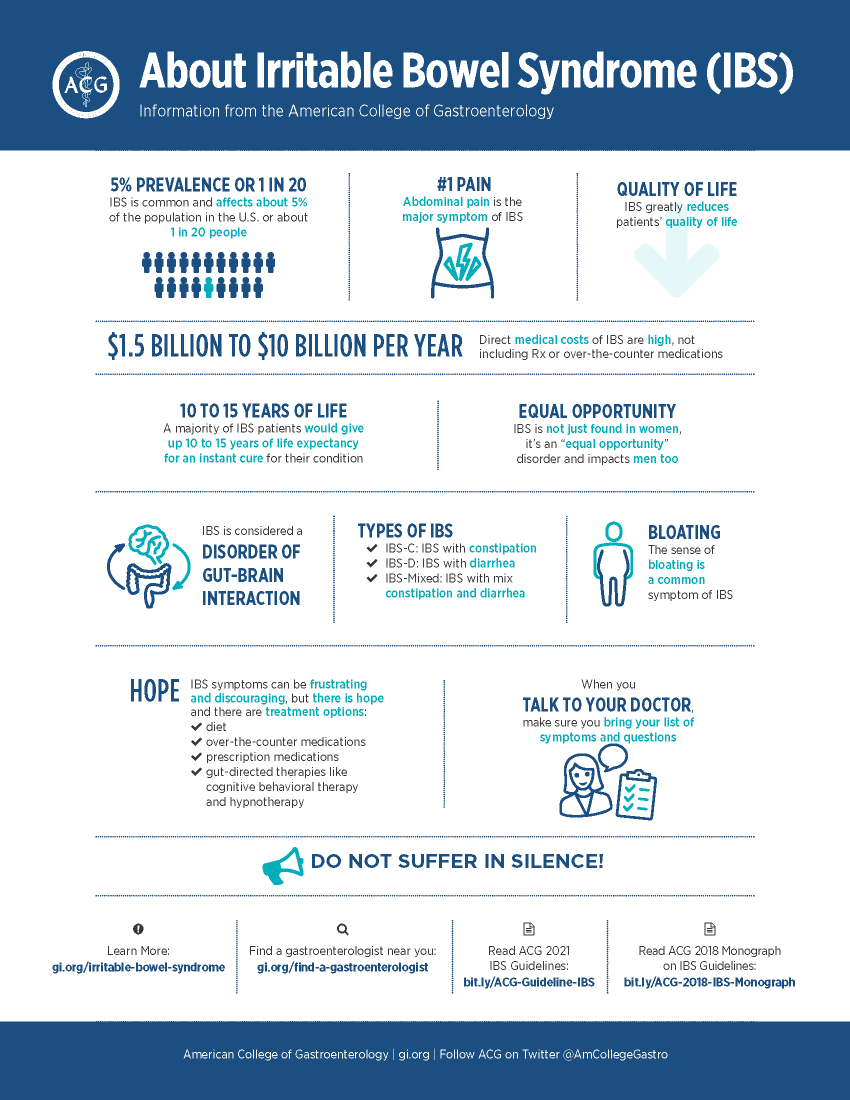
IBS is a widespread and very burdensome disease. It’s essentially a brain/gut dysfunction that is not often being addressed. Significant symptoms show up as abdominal pain, bloating along with diarrhea and/or constipation.
There are three subtypes of IBS: IBS-C, constipation, IBS-D, diarrhea, and IBS-M, mixed. Those three subtypes break-out equally in the IBS population, so you have a third, a third and a third. It’s a chronic condition. 65% of IBS patients are female, ages 25 to 55, and it affects approximately 10-15% of U.S. adults.
Used by Permission © American College of Gastroenterology 2021
Randy Horton:
Wow.
Danny Bernstein:
Yeah, it’s extensive. In the US, there’s about 25 to 45 million people who have IBS.[4] It wreaks havoc on the quality of life. Researchers have found that patients would be willing to give up 10 to 15 years of their life expectancy if they could have an immediate cure for IBS and its associated symptoms.[5]
“…patients would be willing to give up 10 to 15 years of their life expectancy if they could have an immediate cure for IBS….”[5]
People with severe constipation often wind up in the emergency room in severe pain. People with diarrhea have to plan their life around a bathroom. IBS makes life difficult.
Randy Horton:
What a burden in that IBS manifests in such a fairly intimate way. We don’t openly discuss the bowels.
Danny Bernstein:
Very much so. I often talk openly about my own health experiences and quality of life, hoping to normalize the subject around bathroom activities and have others suffering from IBS symptoms seek out help, rather than suffer in silence.
Randy Horton:
I appreciate you giving this background on IBS. A lot of times, when talking about medical devices, people skip right over the prevalence, impact and the human factor. I think that is a mistake. Laying the context is important. I spoke with a regulatory expert at a major medical device firm and he said, “One of the things you want to do is get the regulator enthused about your device, not so that they’ll cheat or cut, but just so that it’s a personal thing.” If they get personally invested in it, they’ll try and find ways to help you get there.
What’s the current state of management of IBS? What’s the void that you saw and the opportunity to fill it or raise the bar?
Danny Bernstein:
The current state of managing IBS is not great. Patients more often than not don’t receive adequate relief from the treatments out in the market. Their physicians therefore are not happy being unable to help their patients satisfactorily live with this chronic condition. This means many more visits from their patients and more attempts at switching prescriptions to find one that helps with the constipation or diarrhea without causing unacceptable side-effects.
When learning about the delivery of the North Carolina protocol by gastro-psychologists at Northwestern Medicine Digestive Health Center, the gap I saw was the limited number of patients who could be treated, and the wait times of a year or more to be treated.
There is a significant body of scientific evidence demonstrating the effectiveness and safety of the protocol in treating IBS. It’s been the gold standard since 1995.[6]
Based on a specific study by the protocol’s author, Olafur Palsson, we had good reason to believe that effective remote delivery was possible. We ran a very small proof-of-concept pilot with Northwestern Medicine Digestive Health Center. Later that year metaMe Health licensed the world-wide rights for the North Carolina protocol. From this point on we developed Regulora as an app, raised money for a large clinical study and received FDA clearance for Regulora as a prescription-only digital therapeutic.
Randy Horton:
Can you describe the actual protocol? What does it do? And what does it look like, in numbers or personal impact, when it is an effective treatment for somebody?
Danny Bernstein:
The protocol itself uses gut-directed hypnotherapy.[7] Seven treatments are delivered every other week over a three month period. Each of these sessions is approximately 30 minutes and the outcomes have been fairly consistent.
Studies have used patient-reported outcomes to measure outcomes of the therapy. In our clinical study, we had 382 patients, and the results were a confirmation that we have a special product in Regulora. You’ll recognize this as I describe some of the metrics.
Some of the top line study results using clinically validated measures showed us that 64% of subjects reported receiving adequate relief of their IBS symptoms; 68% of subjects were satisfied or very satisfied with the therapy; 87% would recommend the therapy to someone with IBS; 90% adherence by patients of their treatment sessions, and 90% would recommend Regulora before trying a prescription drug therapy.
We’re happy about that response from the study population.
Randy Horton:
It sounds like in person treatment from gastro-psychologists is something almost exclusively available at major academic medical centers. If you’re in a rural area, you’re out of luck. And if you live, say, 40 miles in an ex-urban community, you’ve got a long commute multiple times over three months to get the right treatment.
Danny Bernstein:
That is correct.
Randy Horton:
Who administers the traditional treatment? Is it a board-certified hypnotherapist? How does somebody train to do this, and what is the onboarding for somebody to become certified in the protocol?
Danny Bernstein:
There are licensed psychologists who also have training in hypnotherapy and often known as gastro-psychologists. These people are far and few between. We’ve worked with several of the individuals out there, but they’re just not readily available, particularly for the number of people who are suffering from IBS.
Randy Horton:
It sounds like a pretty high bar to be a licensed psychologist and on top of that get a specialization or certification in this specific hypnotherapy protocol. Plus, in order to treat patients, you have to build provider relationships locally in order to create a referral network. It sounds difficult.
Danny Bernstein:
It looks like a tough but very satisfying profession. Keep in mind that gastro-psychologists have years of education and in clinic training. They see patients with a host of conditions and they treat the patient with techniques and therapeutics available as they deem appropriate. metaMe has, thus far, focused on the development of Gut Directed Hypnotherapy in ways to make it as accessible as possible. Today, Regulora is an FDA cleared prescription only digital therapeutic, but it hasn’t always been that way.
When metaMe started out, it was as a telemedicine service. Our goal was, like I said, to make the North Carolina Protocol more accessible. We started offering it via telemedicine and we grew it into six different states. When we heard that the FDA had cleared Pear Therapeutics for their reSET product, we pivoted our approach. We realized that DTx was a more viable way to effectively reach more people. That was the beginning of metaMe pivoting, from the approach that involved therapists in each state and working with patients in a telehealth situation, to a prescription Software as a Medical Device.
Randy Horton:
Working with actual therapists in six states means you have to find a therapist who’s credentialized in the state to treat those patients. In North Dakota, you’ve got to find someone who’s North Dakota-accredited.
Danny Bernstein:
That’s exactly right. Then you work on scheduling between a therapist and their patient. What we were experimenting with was using videos of the therapists, so they would do some live sessions and some recorded sessions. Eventually, the therapists would say to us, “I don’t have time for live sessions. Go ahead and just use my videos.” It was a combination of scheduling issues and reading the same protocol over and over again.
Randy Horton:
It’s not very exciting if you’re a therapist.
Danny Bernstein:
Perhaps not, but it taught us that this could be a perfect job for software.

Randy Horton:
Can you walk me through the patient lifecycle? How does somebody end up using Regulora? How do they find out about it? How does it get prescribed? And where do patients download it – in the app store, I’m assuming?
Danny Bernstein:
It’s in Apple’s App Store and in Google’s Play store. While it’s out there on the marketplace, it’s only available with a prescription. Patients will need a prescription access code to unlock the app and create an account.
At the beginning of the life cycle, a patient will go to their physician and get diagnosed with IBS. Then the doctor can write a prescription for Regulora. That prescription will head off to a specialty pharmacy that will work to reconcile the payments. Then a prescription access code will be given to the patient, and they’ll have any support they need to get going. It’s a downloadable app, and once you download it, it’s a very straightforward self-service, setting up your schedule and following along.
Randy Horton:
If you have IBS, are you going to your primary care doctor or internist to get a prescription? Or are you going to a gastroenterologist?
Danny Bernstein:
Most people start with their primary care physician because of the nature of this condition. It takes people some time to realize that they actually have something that they should be talking to their doctor about. Statistically, 12% of all visits to primary care physicians in the United States alone are for IBS.[8] And for Gastroenterologists, 30% of their visits are for patients with IBS, because of the number of primary care physicians that send their patients to GI docs to see if they can help. These numbers are recurrent, too.
“Statistically, 12% of all visits to primary care physicians in the United States alone are for IBS.”[8]
Randy Horton:
It sounds like, from the point of view of primary care physicians, they’d rather not have all these appointments where they can’t actually help their patients.
Danny Bernstein:
Yes, that’s exactly right. Doctors get frustrated not being able to help patients with the things they come in for. They’re spending a lot of time with these patients and nobody winds up being happy at this point.
Randy Horton:
For traditional therapeutic providers, they usually do outreach and education, supplemented by clinical research, to help physicians understand why their treatment would be something they’d want to prescribe as a new alternative. Presumably, an IBS patient comes in to see the physician, and the physician says, “Hey, there’s a new therapeutic available, and I think you should try it.” Is that the gist of how it works for you?
Danny Bernstein:
It’s exactly how it works. We’re making patients aware of Regulora so they know to ask their physicians, and we’re also going to physicians and educating them about our efficacy, safety, proper usage, type of patient who would be recommended, etc.
Randy Horton:
Even though it might sound weird to a patient for their doc to say, “I have an app that your insurer will pay for that will hypnotize you in repeated sessions and make your condition better.” Though at that point, what does the patient have to lose? Plus, there’s data showing that it works, too.
Danny Bernstein:
I don’t think any doctor’s going to prescribe this without understanding the data. Of all the research that we have done with patients, physicians and payers, they’ve shown a significant interest in Regulora. I think we could even classify their response as a pent-up demand. Pain from IBS is the predominant reason that patients with IBS see their physician, and Regulora is the first and only product specifically indicated for the treatment of abdominal pain due to IBS. We target the primary reason why patients are going to the physician.
“Of all the research that we have done with patients, physicians and payers, they’ve shown a significant interest in Regulora. I think we could even classify their response as a pent-up demand.”
– Danny Bernstein, metaMe Health
Randy Horton:
Just to clarify here, you’re treating one of the worst symptoms of IBS, which is abdominal pain. Does Regulora actually tackle the fundamental structural issues of IBS?
Danny Bernstein:
Yes, it does. It modifies how the brain reacts to signals that the gut is sending it.
Randy Horton:
So the provider says, “I’ve got this app for you.” What happens next? What does the provider do? What does the patient do? Are they talking to their physician throughout, or does the doctor say, “See me in 12 weeks?”
Danny Bernstein:
The physician’s role initially is to establish that someone doesn’t have cancer or Crohn’s. The symptoms can be similar. Once it’s established that what they have is IBS, the patient, with a prescription, is off and running, downloading the app, setting up their schedule.
Then we use patient-reported outcomes to measure progress. There’s a standardized questionnaire, the symptom severity scale (IBS-SSS). We use that to measure outcomes at each of the treatment sessions. At the end, we deliver a report back to the patient that shows baseline, outcomes and adherence. They can share the report with their physician.
In between the three months of the protocol, if the patient has a question, they can reach out to our customer service team or their physician. But the app makes the treatment pretty straightforward. It’s after the treatment that the report comes out and the physician gets involved again. Patients often experience similar results at different points along the pathway of those three months, but it is not always consistent. A physician does not need to engage over the course of the three months.
Randy Horton:
You prescribe it and they’ll hear about it at the end, basically.
Danny Bernstein:
Yes.
Randy Horton:
How do you know if a patient will actually use your DTx? Just because you give someone a digital therapeutic, it doesn’t mean they’re actually going to use it. How do you move the needle on that? How do you figure out the whole problem of adherence?
Danny Bernstein:
That’s one of the great advantages of a digital therapeutic – we have an exact record of every time a patient interacts with the app. So, unlike a drug, we can precisely track adherence in an objective way.
As far as getting people to use Regulora, all the research we’ve done with patients in the clinical trials indicate satisfaction and feeling of improvement. It really is a reinforcing virtuous cycle – with 90% adherence to these treatments, you wind up with a treatment that has nominal side effects, demonstrates improvements and makes people hopeful. They experience improvements because they’re following the protocol, which is what they’re supposed to be doing. We anticipate that people in the real world who receive a prescription will follow through, too.
Randy Horton:
One of the upshots of such an awful chronic condition is that you have an extremely motivated patient base.
Danny Bernstein:
Think about your gut as a sensory organ that you experience through food or through situations. You walk into your boss’s office, you’re afraid you’re going to have a difficult conversation, and the next thing you know, you have urgency to go to the bathroom, you’re having abdominal pain, etc.
These are the types of signals that are getting sent to your brain and then your brain is reacting. People with IBS are oftentimes referred to as being viscerally hypersensitive. They’re overly sensitive to these signals, and this is where the dysregulation between the brain and the gut comes in and leads to flare-ups.
Randy Horton:
Who pays for this app?
Danny Bernstein:
We believe that insurers will want to pay for this when they see the reductions in symptoms and the savings to their bottom line. Obviously, insurers care about their bottom line – they want to see how a new treatment is going to benefit not only the population, but also their finances. And this is a treatment with good outcomes, nominal side effects and that doesn’t cost as much as traditional drugs.
Gut-directed hypnotherapy has been around since 1984, and there are 10+ randomized control trials (RCTs) showing its effectiveness. There are 20 other studies that are not RCTs that show various types of benefits from these treatments.
Randy Horton:
The inventors of this protocol, and the people who have been the strong proponents in these academic medical centers, must be just totally thrilled that you’ll be able to scale this, right?
Danny Bernstein:
Yeah, I think so. We’ve had tremendous amounts of advice and input from various people at the university level, including Dr. Lin Chang at UCLA, and the 34-person proof of concept study with patients from the University of Chicago, Northwestern, Harvard, UPENN, UCLA and Stanford. These are the institutions currently working in this space and are interested in whether there’s benefit in this model. They were receptive to our conversations and supportive in our research. I anticipate that in the marketplace, physicians will be welcoming of an alternative to what they have to work with today.
Now that you’ve familiar with Regulora, and the incredible potential it has to improve the lives of millions of patients with IBS, you may be interested in how metaMe went from a great concept to an actual FDA-cleared DTx. In Part Two of this interview, Danny discusses the people who helped propel metaMe to success with industry experience, investment acumen and belief in the product.
This is Part One of a two-part interview between Orthogonal’s Randy Horton and Danny Bernstein of metaMe Health.

References 1. Dr Faubion B. Crohn's disease - Symptoms and causes. Mayo Clinic. https://www.mayoclinic.org/diseases-conditions/crohns-disease/symptoms-causes/syc-20353304. Published 2022. Accessed May 5, 2022. 2. Irritable bowel syndrome - Symptoms and causes. Mayo Clinic. https://www.mayoclinic.org/diseases-conditions/irritable-bowel-syndrome/symptoms-causes/syc-20360016. Published 2021. Accessed May 5, 2022. 3. Palsson O. Standardized hypnosis treatment for irritable bowel syndrome: the North Carolina protocol. PubMed. https://pubmed.ncbi.nlm.nih.gov/16316883/. Published 2006. Accessed May 5, 2022. 4. IBS Facts and Statistics - About IBS. About IBS. https://aboutibs.org/what-is-ibs/facts-about-ibs. Accessed May 5, 2022. 5. Canavan C, West J, Card T. Review article: the economic impact of the irritable bowel syndrome. PubMed. https://pubmed.ncbi.nlm.nih.gov/25199904/. Published 2014. Accessed May 5, 2022. 6. Palsson O, van Tilburg M. Hypnosis and Guided Imagery Treatment for Gastrointestinal Disorders: Experience With Scripted Protocols Developed at the University of North Carolina. Taylor & Francis. https://www.tandfonline.com/doi/full/10.1080/00029157.2015.1012705. Published 2015. Accessed May 5, 2022. 7. Hypnosis - Mayo Clinic. Mayoclinic.org. https://www.mayoclinic.org/tests-procedures/hypnosis/about/pac-20394405. Published 2020. Accessed May 5, 2022. 8. IBS Facts and Statistics - About IBS. About IBS. https://aboutibs.org/what-is-ibs/facts-about-ibs/statistics/. Accessed May 5, 2022.
Related Posts

Article
Roundup: Bluetooth Medical Devices Cleared by FDA in 2024

Article
Help Us Build an Authoritative List of SaMD Cleared by the FDA

Article
SaMD Cleared by the FDA: The Ultimate Running List

Article
Roundup: Bluetooth Medical Devices Cleared by FDA in 2023