
Article
Patient Engagement & UX for Bluetooth Medical Devices
This blog contains Chapter 2 of the Orthogonal white paper titled: Accelerating the Creation of Software as a Medical Device (SaMD) with Product Analytics. The following are links to each chapter of this white paper:
Product analytics information is consumable without the need for highly specialized skills. This allows a range of professionals to consume the data, such as product management, design, and marketing. It can be used during initial design/development, clinical trials, and in production once the product is formally launched.
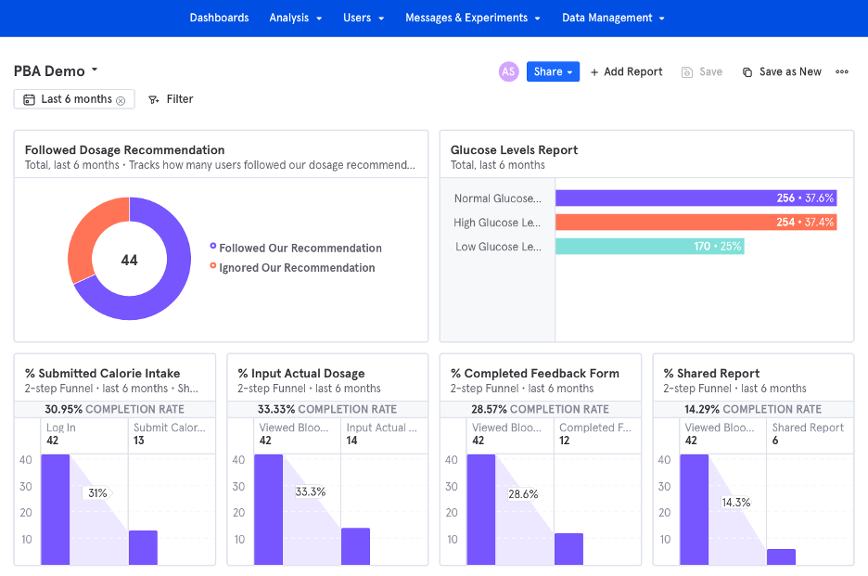
The following is an example of a product analytics dashboard implemented with Mixpanel that shows the power of user data viewed at aggregate levels for individual users and/or at cross-user levels. Custom dashboards allow various groups such as R&D and marketing to see exactly how users are using the product in real-time:
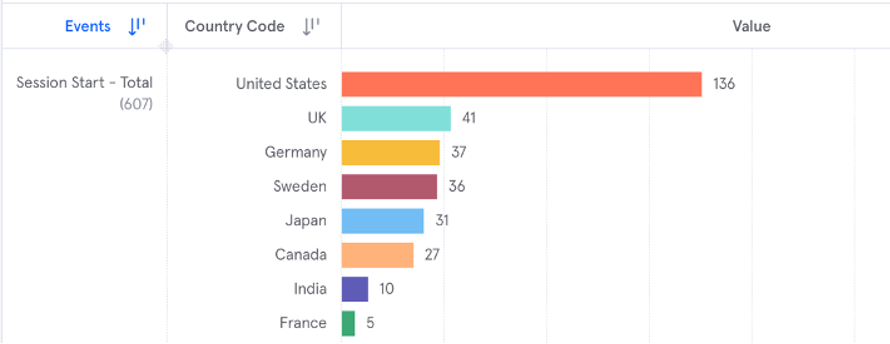
The below example is of a product insights report (implemented with Mixpanel) that allows the device manufacturer to track how many people by country are using the software in a given time frame. Product analytics tools usually come with a variety of out-of-the-box reporting options based on industry best practices, as well as customizable reports. Reports can also typically be exported so that they can be used as part of a cross-system data warehouse or analytics tool. These reports can be generated and modified by analysts who do not have any programming skills.
There is a rich marketplace of product analytics tools to select from including:
At Orthogonal, our go-to tool for product analytics with connected medical devices is Mixpanel. After assessing several tools in the marketplace over 2019 and early 2020, we found Mixpanel to be a good fit for connected medical devices:

You might ask what the alternatives are to implementing off-the-shelf, person-based analytics using a tool such as Mixpanel. We’ll be blunt in our answer, so that you can spend your time making new and interesting mistakes and not old and proven ones that our team has already learned:
We would like to point out some best practices for implementing and using product analytics:
We would also like to point out some worst practices that should be avoided:
If this white paper has piqued your interest in this topic, and you are wondering what would be involved in adding product analytics to your medical device toolkit, we recommend the following these steps:
We’ll close with two final takeaways on this topic:
If you do go down this path, we’d love to hear from you directly (or through an anonymous email account) so that we can share ideas and best practices. In our estimation, product analytics is far too important to the health of each of our family members, friends, neighbors, and co-workers to not help each other spread this value across every connected medical device. Hopefully, you agree with us that this kind of real-time data provided can enable insights that lead to more rapid conceptualization and turnaround of new features.
Orthogonal is a software developer for Software as a Medical Device (SaMD), digital therapeutics (DTx) and connected medical devices.
We work with change agents who are responsible for digital transformation at medical device and diagnostics manufacturers. These leaders need to accelerate their pipeline of product innovation to modernize patient care and gain a competitive advantage.
Orthogonal applies deep experience in SaMD and the power of fast feedback loops to rapidly develop, successfully launch, and continuously improve connected, compliant products—and we collaborate with our clients to build their own rapid SaMD development engines.
Over the last 10 years, we’ve helped a wide variety of firms develop and bring their regulated/connected devices to market.
Almost twenty years ago, we began our consulting work to create great software products in a range of industries, including financial services, education, research, and healthcare. We spent nearly a decade working with leading-edge fast feedback loop techniques that have now become recognized best practices such as Agile software development (with XP, Scrum, and Kanban), Lean User Experience (Lean UX), and Lean Startup.
Nearly a decade ago, we got a glimpse of digital healthcare and the enormous potential of the cloud, IoT, and smartphones, and the potential impact of medical device software. Realizing that’s what we wanted to focus our energies on exclusively, we narrowed our focus to the development of SaMD, DTx and connected medical devices.
We’ve never looked back. Our team gets up every day excited to help move the needle on healthcare. For almost ten years, we’ve helped a wide variety of firms develop and bring their regulated/connected devices to market. We’re even more excited about the next ten years and what we will help accomplish during The Great Acceleration of Digital Health.
If you need help building your next connected device, or to learn more,
send us a message or call us at (866) 882-7215.
Website: https://devorthogonal.wpengine.com/
Twitter: @orthogonal_io
Our favorite inbound messages after we publish a white paper do not start with, “Great white paper. Keep up the good work.” Our favorites are the ones that start with, “I read your white paper and I disagree when you say X because you are not taking Y and Z into consideration.”
Our ideas are ultimately strengthened by open, candid conversations. We’d love to hear from you. Do you agree with our take? Disagree? Have questions? Have you read an article that we should read? Feel free to reach out to Orthogonal’s CEO, Bernhard Kappe and Randy Horton, Orthogonal’s VP of Solutions and Partnerships.
In return, we will look for ways to share more white papers, personalized insights, content, and introductions that are highly relevant to your work.

This blog contains Chapter 2 of the Orthogonal white paper titled: Accelerating the Creation of Software as a Medical Device (SaMD) with Product Analytics. The following are links to each chapter of this white paper:
Related Posts

Article
Patient Engagement & UX for Bluetooth Medical Devices

Article
How Design Can Improve Ratings for Medical Device Apps

Article
5 Keys to Integrating UX Design With Agile for SaMD
Article
Accelerate Your SaMD Pipeline with Product Analytics