
Talk
Webinar: Shift Left in SaMD Development
Randy Horton:
Hello, and good morning. I’m Randy Horton, Vice President of Solutions and Partnerships at Orthogonal. Also here today and co-organizing this webinar is Bernhard Kappe, Orthogonal’s CEO and founder.
Thank you all for joining us here today.

Randy Horton:
We also want to welcome our All-Star lineup of panelists and speakers. They’re all in key leadership roles at some of the best-known companies in MedTech today, where they do very impressive and important work.

Randy Horton:
Today’s agenda is 90 minutes long, but we’re going to move at a pretty rapid clip. Our goal is for you to learn a lot and not get bored. We’ve divided our four sets of speakers into two major domains. First, we’ll look at how to accelerate software development for medical devices, starting with a higher level view of it, and then drilling down to a case study. Then we’ll look at the parallel question of how to accelerate AI development for medical devices, starting broadly and again going into a case study. We’ll wrap up with an audience question and answer (Q&A) session.

Randy Horton:
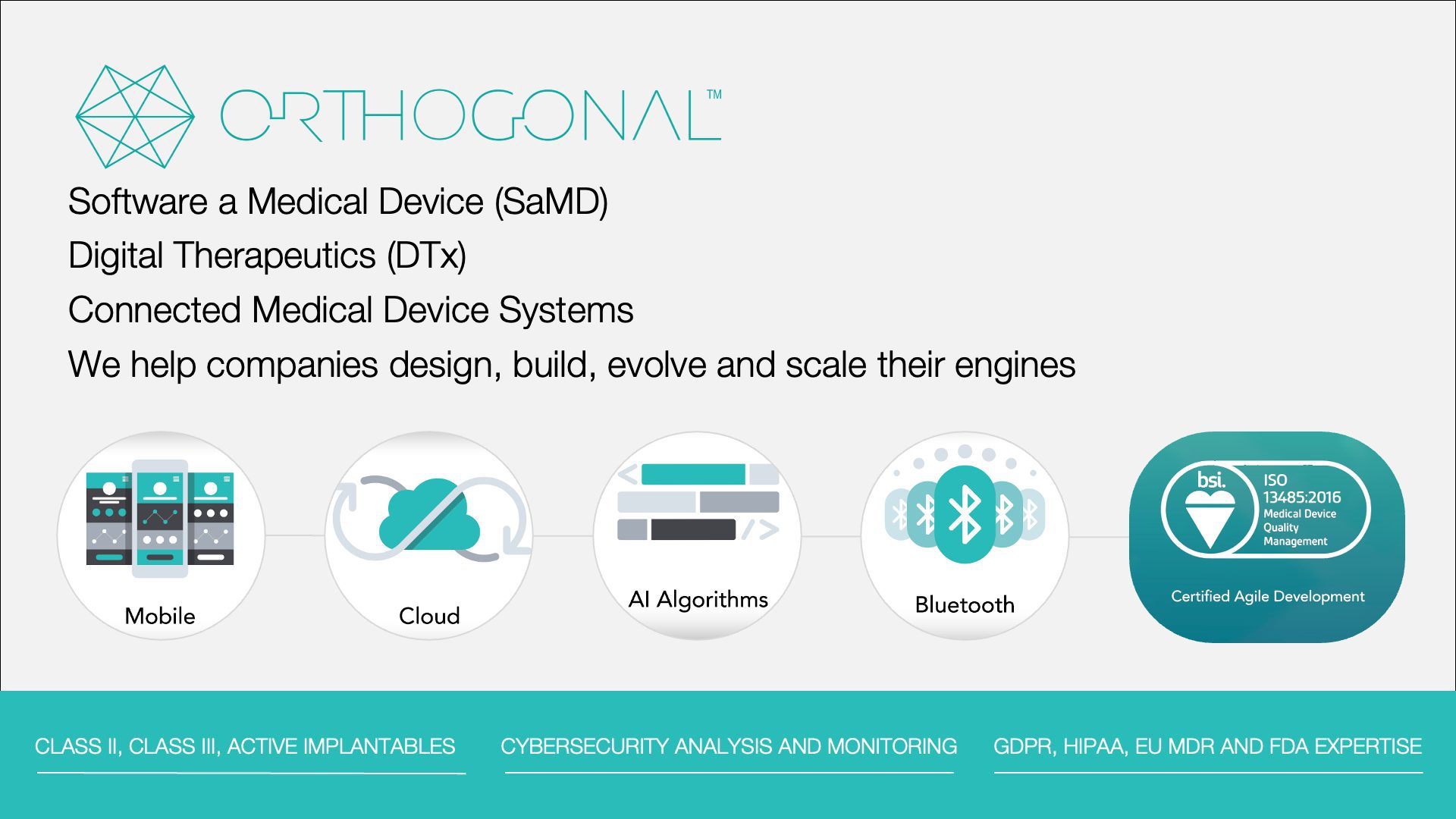
Just a very short introduction about our firm: Orthogonal is a software consulting firm that works with medical device manufacturers, diagnostics makers and pharmaceutical companies to help them accelerate their design and development of connected medical devices, Software as a Medical Device (SaMD) and Digital Therapeutics (DTx). These categories encompass all the pieces of software that are regulated as part of a medical device that’s brought to market, but are not physically located on the medical device. We work on a variety of devices, including Class II, Class III and active implantables.
Randy Horton:
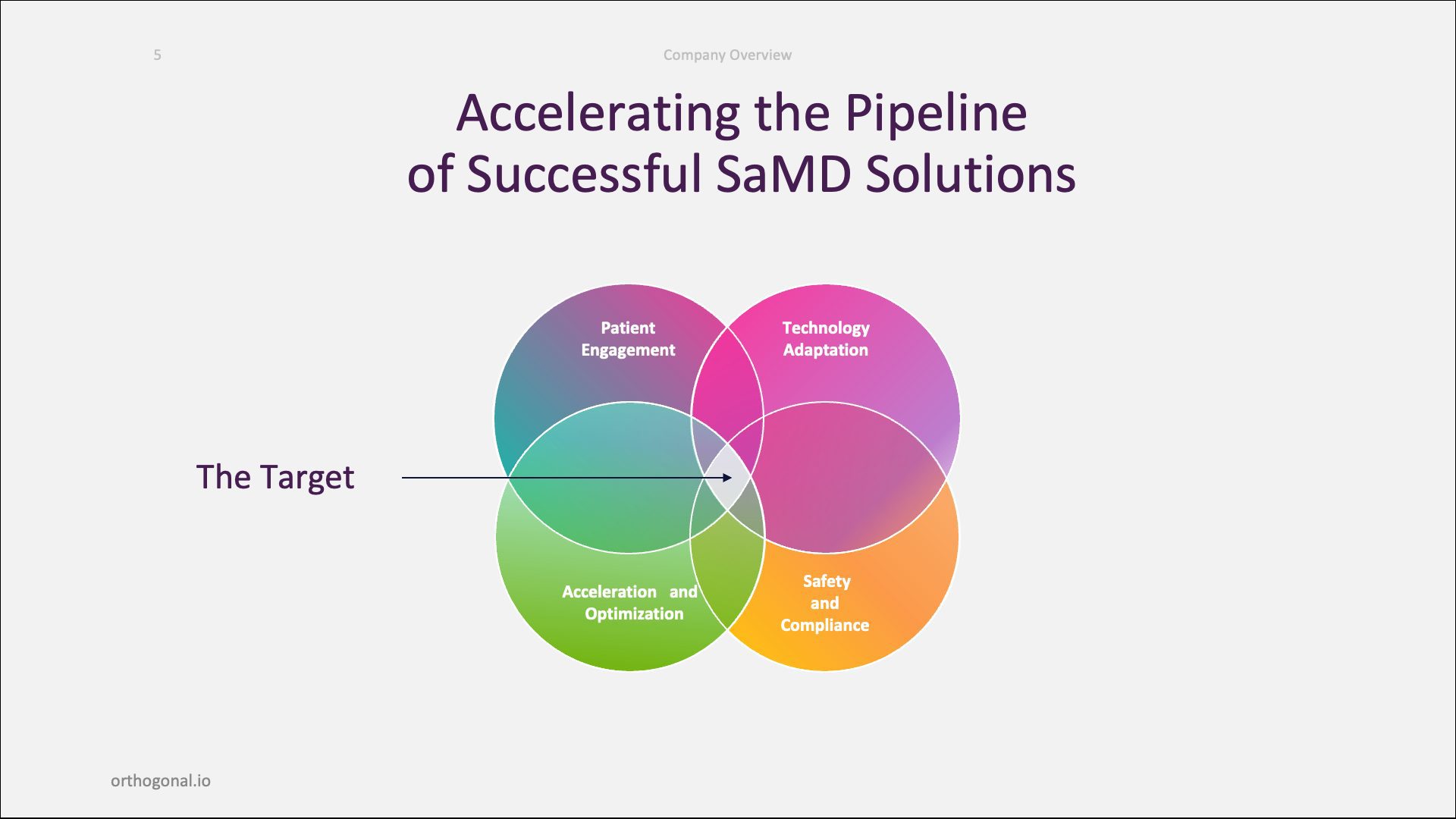
Post-launch, we help these same companies evolve and scale up their software. Finally, we help our clients build scalable development engines for themselves so that they can have internal SaMD factories.
Our work sits at the intersection of four key domains: patient engagement, technology adoption, acceleration and optimization and, of course, safety and compliance.
Now, I’m going to ask Bernhard and Michael to take a minute and introduce themselves.

Bernhard Kappe:
I’m Bernhard Kappe, I’m the founder and CEO of Orthogonal. I’ve been working in the connected device space for 15 years or so. Prior to that, I worked in software and a number of other areas. As Randy mentioned, at Orthogonal we’ve been knee-deep in this intersection of what you’d call “Software is Eating the World” and the medical device space. We’ve been wrestling with the question of how to get better and how to do that faster – as tech companies do in other industries – while maintaining patient safety and compliance in a slowly evolving regulatory landscape.
I’m really excited about our speakers today. They are folks we’ve collaborated with and shared ideas back and forth with to answer some of these questions. We’re eager for them to share some of their thoughts on the subject. There’s a lot we can learn from each other and a lot that we will share today.
We hope this is the beginning of an ongoing collaboration with interested, highly-experienced professionals.
Michael Iglesias:
My name is Michael Iglesias. I just joined Roche this week. I got started in this industry working in mobile technology. I was working at Apple when they launched the first generation iPhone and the first generation iPad. That’s when I knew that these kinds of devices were really going to change our behavior, and everything we knew about technology was about to change. From there, I went into the MedTech industry – actually with Carl Washburn at Janssen Pharma within J&J. Since then, I’ve been at Eli Lilly, Phillips, Novo Nordisk and now Roche. My focus has been mobile apps, cloud technology, and even some physical connected medical devices.
Randy Horton:
Thank you, Michael. We’re thrilled to have you today. The breadth of your experience across this industry is something that really stands out and I know that you’ve got some great insights coming now for our attendees.
To quickly build on what Bernhard was saying: There’s a lot of people dreaming about the power of connected medical devices and digital medicine. But there’s a much smaller group of people who have actually put serious medical devices that are connected – ones with tremendous power and tremendous risk – into the market.
Today’s Goal

Randy Horton:
Our All-Star lineup of speakers today all have products on the market with their companies’ names on them. They’ve learned hard lessons along the way as the first ones making these strides into the market, so to speak. We’ll be hearing their best practices, worst practices and case studies on how to accelerate the development of medical device software, particularly connected software. Our goal is to cram about 10 hours of great content into 90 minutes.

Randy Horton:
As Bernhard alluded to, it was 10 years ago this August that Marc Andreessen penned his famous Wall Street Journal op ed, where he said, basically, “Software is eating the world in all sectors. And in the future, every company will become a software company.”[1] It was a pretty bold statement then – somewhat controversial, even. But I don’t think anybody is debating his point now.
As we’ve seen in MedTech just over the last decade, an increasingly large share of the value created by our solutions is not coming from biology, chemistry or physics, but rather from the software, the data and the software’s ability to simulate and interface with biology, chemistry and physics.
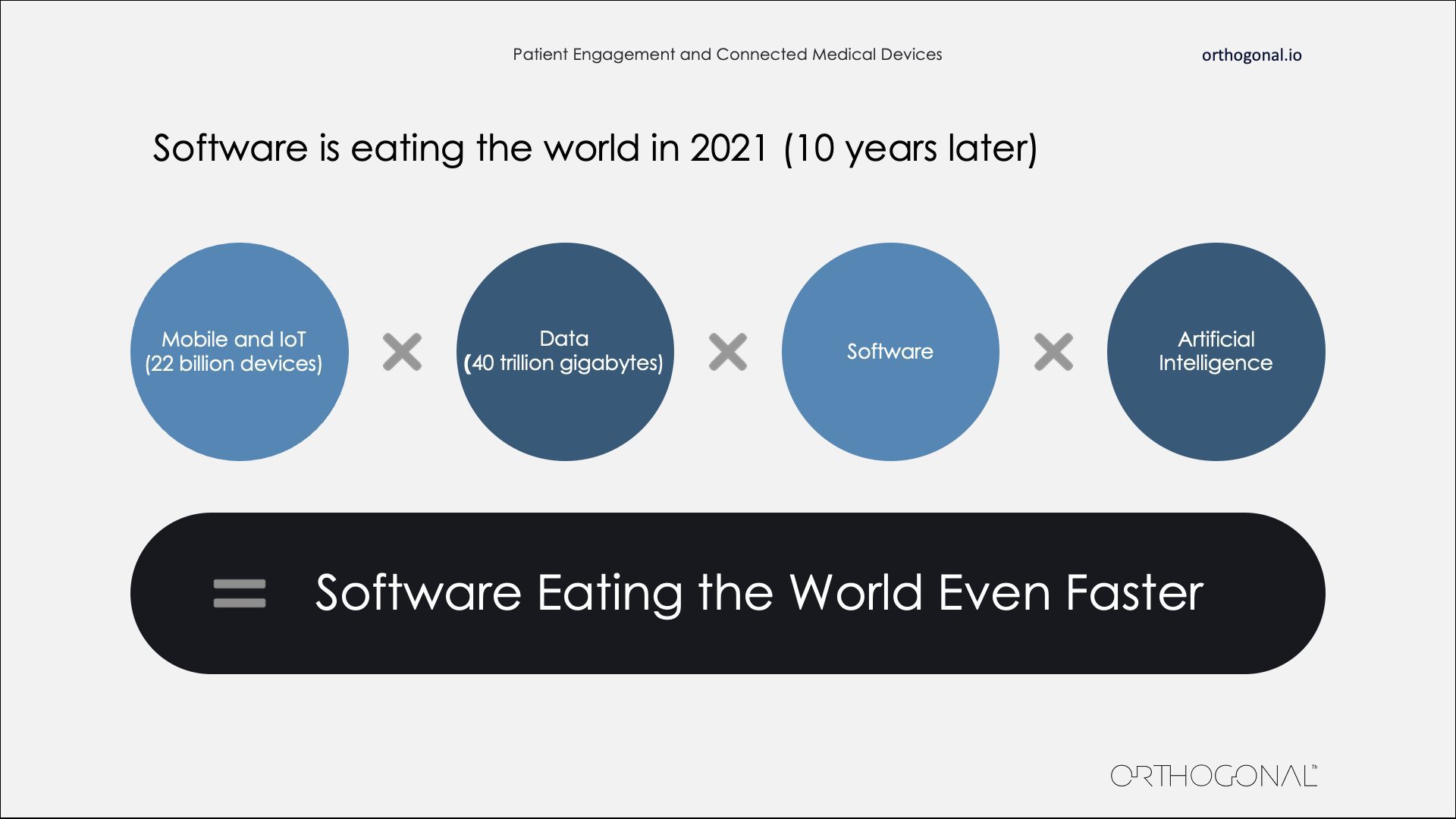
If anything, software is eating the world even faster.
Software is Eating the World Even Faster
Randy Horton:
I want to touch on the biggest contributing factors to that acceleration. To get the obvious one out of the way: Everybody has a smartphone. There are more active smartphones out there than there are people in this country. When you throw IoT devices in that count, you’re somewhere in the trillions of gigabytes of data. We have tremendously powerful AI algorithms, tools, techniques and hardware that sift through that data to separate the signal from the noise. We can identify the insights that can change and change behavior just by leveraging the data that our devices create. All of this is important if we want to move the needle on healthcare outcomes and bend down the healthcare cost curve.
An Iterative World
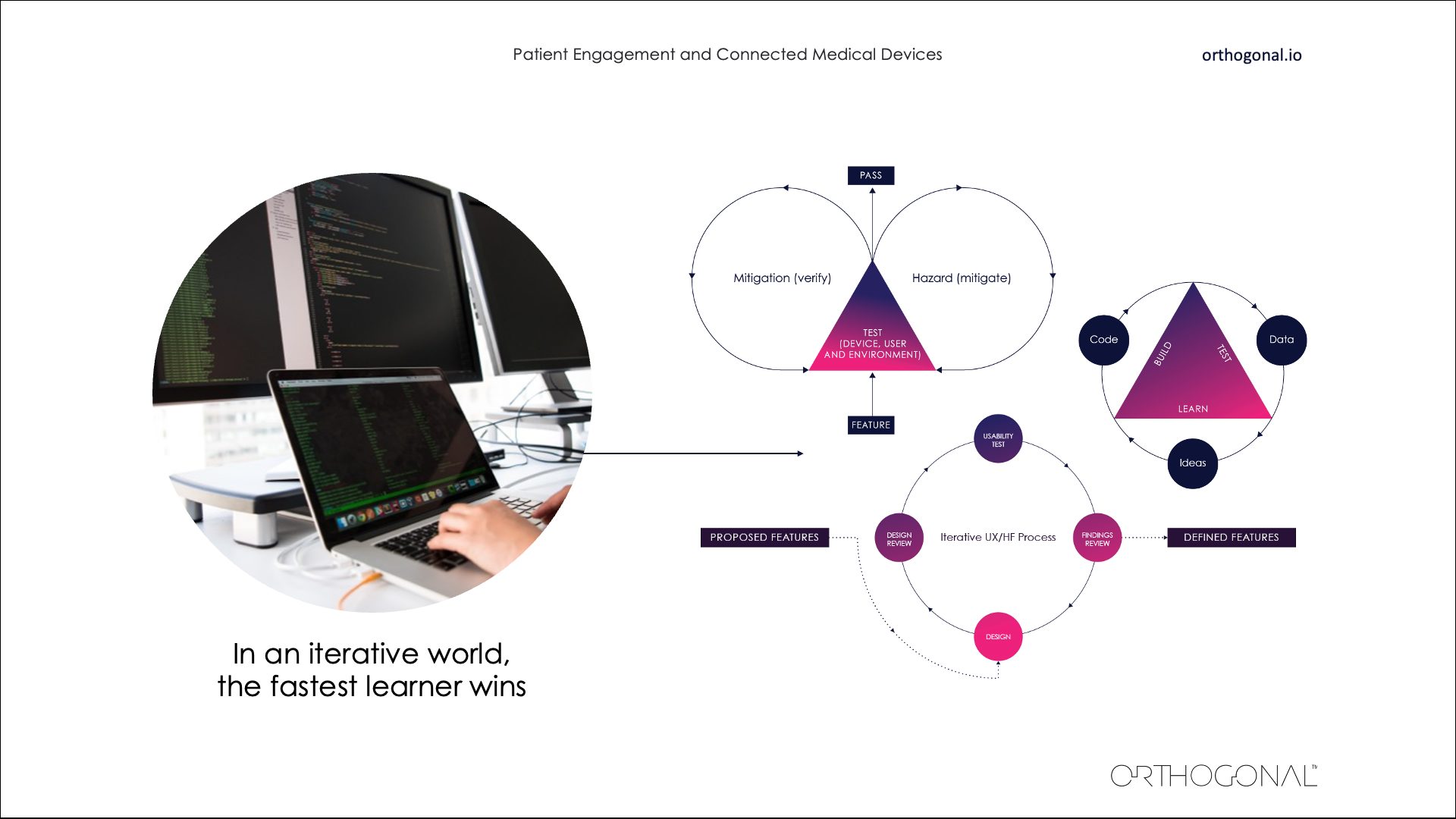
Randy Horton:
Another factor is Big Tech. It’s been really impressive, if sometimes a little disturbing, to watch how quickly these tech giants – in all industries – have been able to learn and evolve and improve. They’ve really got it down to a science. Everything is about fast feedback loops in iterative learning, conducting small tests and seeing what works and what doesn’t work. They’re continually making better products and getting faster with their processes. And in an iterative world, the fastest learner is the one who wins. The more success you have, the more money you make. And the more money you make, the more you can invest back in R&D to pull even further ahead of the competition.

Randy Horton:
Big Tech is growing rapidly using a set of core techniques. They learn fast. They avoid making assumptions. And they’re willing to make mistakes early on when it’s cheap to do so.
Randy Horton:
Facebook was famous and now (probably) infamous for this expression: “We’re going to move fast and break things.” It’s a cavalier approach, and not at all what we do or are ever going to do in medical devices.
We’re not like Facebook. We have a responsibility to our patients. Snapchat is not an implanted device in the heart or on the spine, and Netflix is not an insulin pump. If we move fast and break things in our space, we’re breaking human bodies. Not only that – with connected medical devices, we run the risk of breaking bodies at scale. That’s a frightening prospect.
We also have more roadblocks and constraints to contend with in this space. Other industries have to deal with regulatory oversight, but not necessarily to the extent we do. We have numerous constraints because the things we build and distribute have to demonstrate safety, effectiveness and regulatory compliance. There are processes and regulatory filings and generally lots of extra time and effort we have to put in to prove that we know what we’re doing.

Randy Horton:
Sometimes, people in this space can despair, and say, “Gee, are we Gulliver, tied down by thousands of regulatory Lilliputians? It’s not just one thing holding us back. It’s this mass of little things tying us down that keep us from ever moving faster.”


To that, let us be the one to say: That’s not our destiny. You’re going to hear today about the middle ground where you can move faster and break nothing. We actually liked this expression so much, we’ve printed it on hoodies for our staff.
Our evidence that this middle ground exists and can thrive comes in the form of what our speakers will share today. They’re going to talk about bringing serious devices and other solutions to market in faster and faster ways. You’ll learn how they’ve leveraged modern engineering methods, modern software techniques and modern business models while still providing documented safety and efficacy.
We’d argue that our speakers have raised the bar on what’s considered safe and effective, and how we can prove that to regulators and patients alike.
Before we jump over to Carl and Don from Eli Lilly, Michael and Bernhard, do you want to add anything?
Bernhard Kappe:
No, I think you’ve summarized it quite well. I’m looking forward to some of the questions after the presentations.
Randy Horton:
Michael, my only question would be this: Did you predict all of this when you were working on the first iPhone?

Michael Iglesias:
No, definitely not. But I remember back in 2007, when I had the iPhone in my hands and got to play with it before the public did. It was one of those moments where you think, “This is definitely going to change things across every industry and landscape.”
Below is a list of the other sections of our Move Faster & Break Nothing Webinar:
Reference 1. Andreessen M. Why Software is Eating The World | Future. Future. https://future.a16z.com/software-is-eating-the-world/. Published 2011. Accessed January 3, 2022.
Related Posts

Talk
Webinar: Shift Left in SaMD Development

Talk
What Medical Device Software to Develop Under QMS: Webinar Summary

Talk
Sizing up SaMD Projects for Success: Webinar

Talk
3 Tools You Need for SaMD App Development: Webinar